US Government COVID-19 Genetic “Vaccine” Databases: Sloppy or Corrupt? Or Both?

Defense Military Epidemiology Database (DMED), Vaccine Adverse Events Reporting Systems (VAERs), and v-safe have numerous irregularities. Responsibility diffusion and secrecy have been employed to protect those involved.
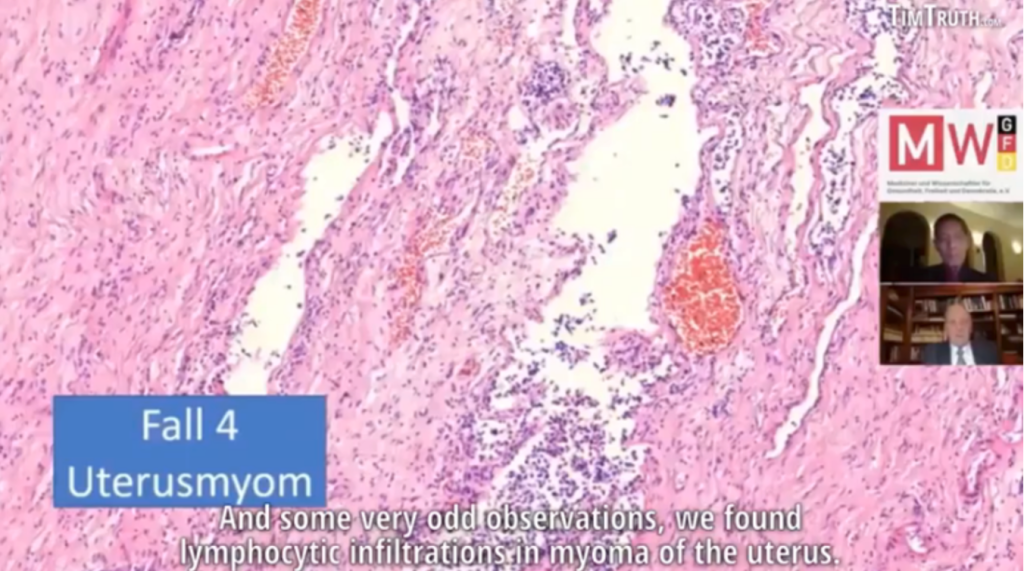
Lymphocytic infiltration of the myometrium (muscle layer) following COVID-19 spike-producing drugs. (Burkhardt Group Collection)
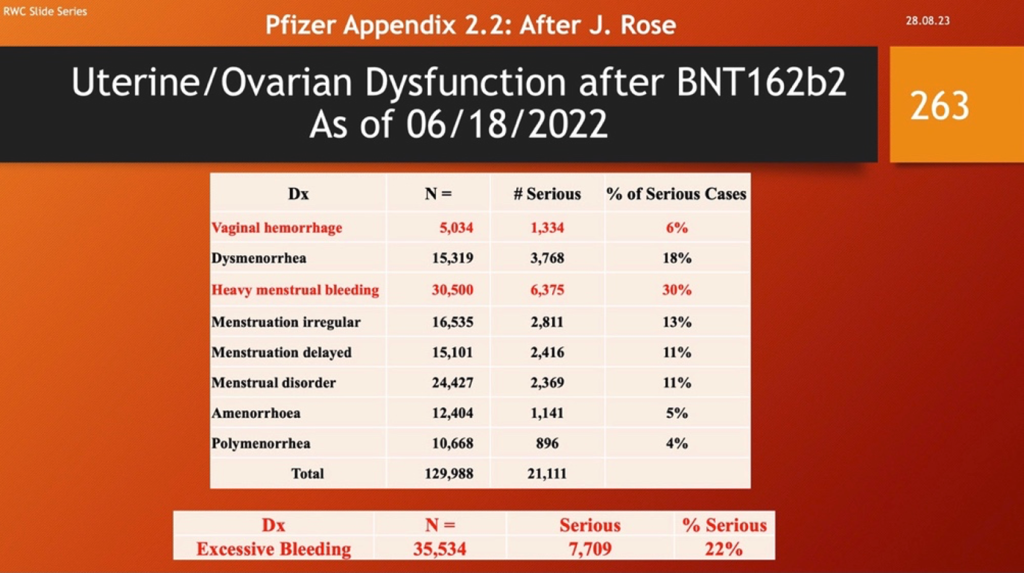
It is not surprising to observe the consequences of this battle in the uterus including 130,000 reports of menstrual disorders, including over 35,000 cases of excessive bleeding. But this number may only be a small percentage of the real number and points to the inadequacy of the government surveillance systems.
COVID-19 mRNA “vaccines” are experimental drugs deliberately designed to rapidly migrate from the deltoid muscle, where they are injected, into both the vascular and lymphatic systems. A specially designed transport vehicle of lipid nanoparticles was created to distribute the mRNA throughout the body, including across the blood-brain barrier into brain and through placenta to the fetus. The photomicrograph above shows lymphocytes infiltrating the uterus on their hunt for non-host proteins.
The genetic material is released in the tissues, gains entry to cells, and then directs the host cells to manufacture proteins that have not been thoroughly characterized but are recognized as being non-host (i.e., not a natural part of the body). These proteins, including spike protein, then initiate an immunological response. Anti-spike antibodies are also produced.
Quality control has been awful with the manufacture of these highly variable products. Toxicity of these concoctions has varied from subjects who die in the chair where they have been injected, to rapid-onset, dramatic cases of cancer, clotting, bleeding, sudden death, and strokes. More subtle manifestations include skin rashes and slow-onset protein deposition disease. The contents of some vials have been shown to be inactive.
An experimental, genetically active drug with known toxic byproducts, widely distributed in the body, calls for extraordinary surveillance after it is released. Sadly, this has not happened. Pfizer’s randomized, controlled trial was unblinded less than four months into public rollout of the “vaccine,” thus destroying the most powerful tool to understand the consequences of this poorly studied treatment.
Next, descending the quality of the science ladder, come prospective studies with matched controls. Vaxxed, unvaxxed, and Phase 2/3 trial crossover subjects would be followed for at least two years under a strict study protocol. [War Room/DailyClout.io Report 82 documents the complexity of this subgroup. The protocol deviation cases need separate analysis as do the gap cases. Those people who have adverse events in the first 21 days after injection may have special vulnerability during that time due to immunosuppression.]
Fatalities after treatment with the experimental gene therapy drugs should receive a detailed autopsy according to the method of the Burkhardt Group. Sampling methods applied to prospective autopsy studies should have been employed in a systematic manner. This did not happen.
Further down the scale of scientific rigor come the retrospective studies, small series, and case reports. These observational type studies can yield valuable clinical insights that help design additional research.
The lowest rung on the research ladder is what is known as a registry. This method entails passive data collection from subjects, medical providers, and interested parties. Very little medical information is recorded in a registry. Registries are used to detect warning signals that then trigger more rigorous study.
In this article, three United States government registries will be evaluated: v-safe managed by the Centers for Disease Control and Prevention (CDC), the Defense Medical Epidemiology Database (DMED) managed by the Defense Medical Surveillance System (DMSS), and the Vaccine Adverse Event Reporting System (VAERS) maintained by both the CDC and Food and Drug Administration (FDA).
1. V-Safe database shut down. It is now gone. It should have run in perpetuity in order to track the potential long-term complications from the experimental gene therapy products.


Realistically, this is not a big loss because VAERS continues. Furthermore, the plethora of warning signals in DMED and VAERS have been ignored anyway, such as the myocarditis signal.
The information below is from Pfizer’s document 5.3.6 post-marketing report summarizing adverse events during the first 10 weeks in the US and the first 12 weeks in the UK. 57 cases of myopericarditis were identified.
Amazingly, they appeared under the heading of autoimmunity instead of under cardiovascular with the other cardiac disorders identified after BNT162b2. Someone, maybe many, recognized autoimmune myocarditis following BNT162b2 in very early 2021.

Note the ubiquitous, “Conclusion: This cumulative case review does not raise new safety issues. Surveillance will continue.”
2. DMED (DoD medical database). This database had the potential to be a valuable tool for tracking the known harms as well as new cases of the myriad manifestations of CoVax disease due to the captured military population it records.
Unfortunately, problems surfaced with this database when past years were “trued up” to line up with 2021, a low-COVID, high-“vaccination” year. The DoD claimed this true up was a correction of a “glitch” of some kind. [https://www.ronjohnson.senate.gov/services/files/2C840675-F495-448C-9C32-A441E1DD45E6]
Others concluded the true up was in reality a “Data Massage” – i.e., adjusting past numbers to make current, post-vaccine complications look better with partial year 2021 data. The following slides illustrate how the DMED data changed. The data from 2021 covers a partial year.
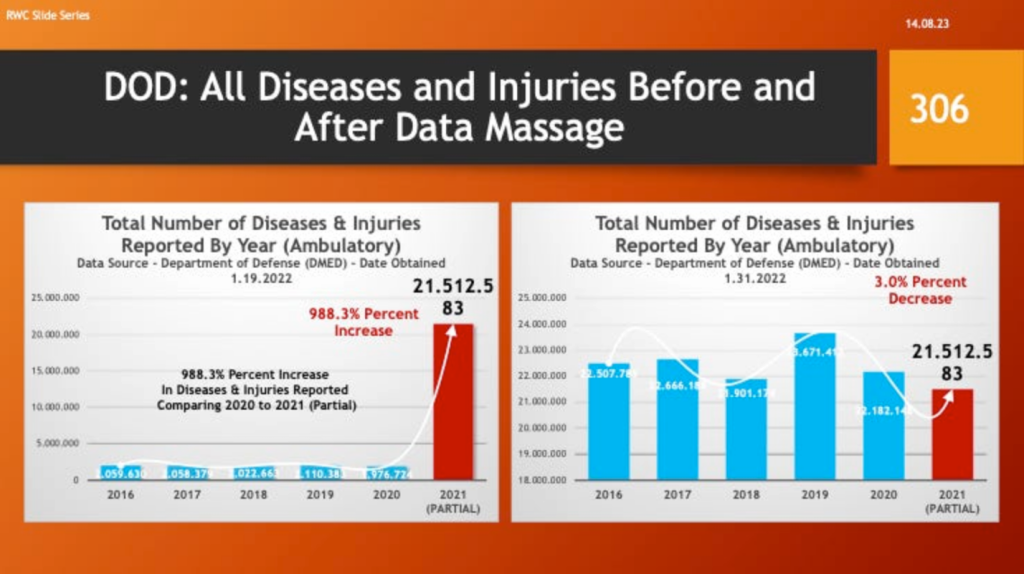
All ambulatory diseases and injuries went from an increase of 988% to a 3% increase.
All diseases and injuries requiring hospitalization went from an increase of 37% to a 14% decrease.
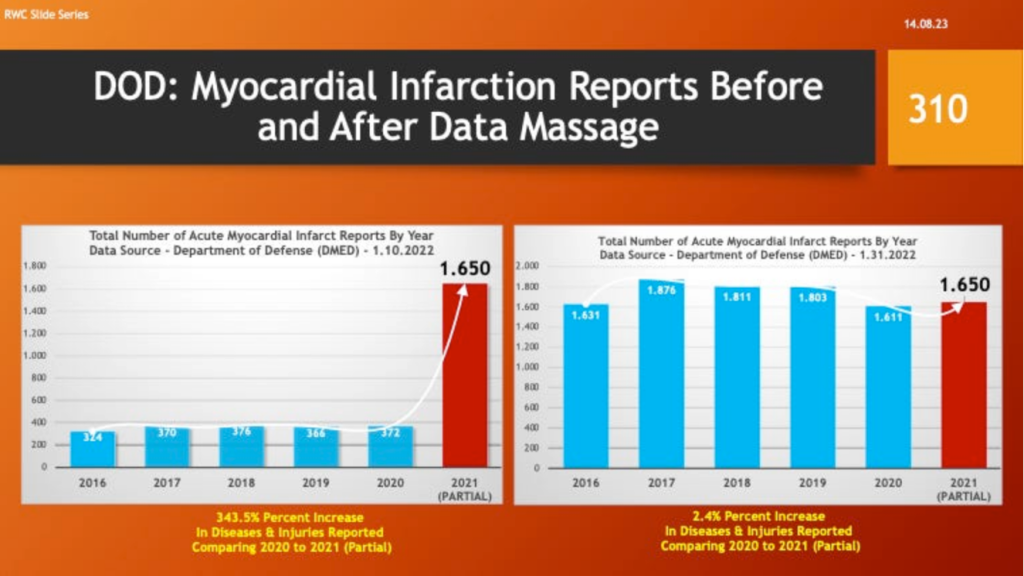
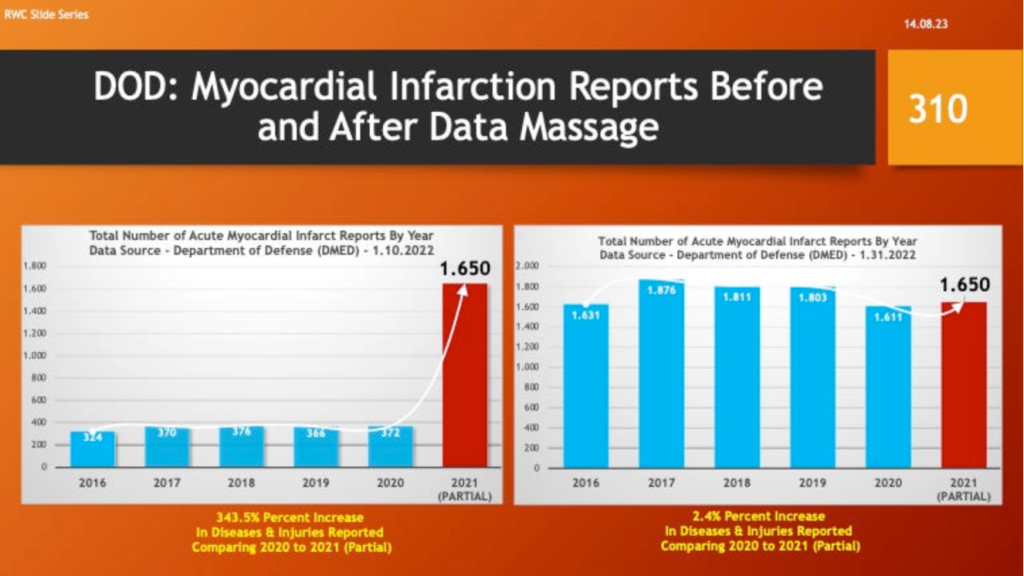
Myocardial infarction reports went from an increase of 344% to a 2.4% increase over 2020 and a decline from the five-year average.
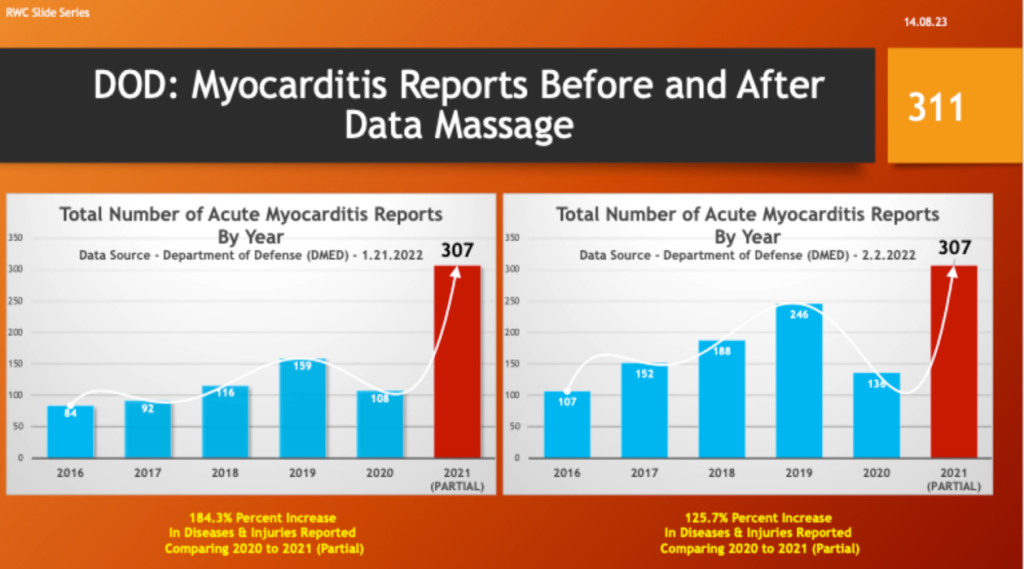
Myocarditis reports went from an increase of 184% to a 126% increase over 2020.
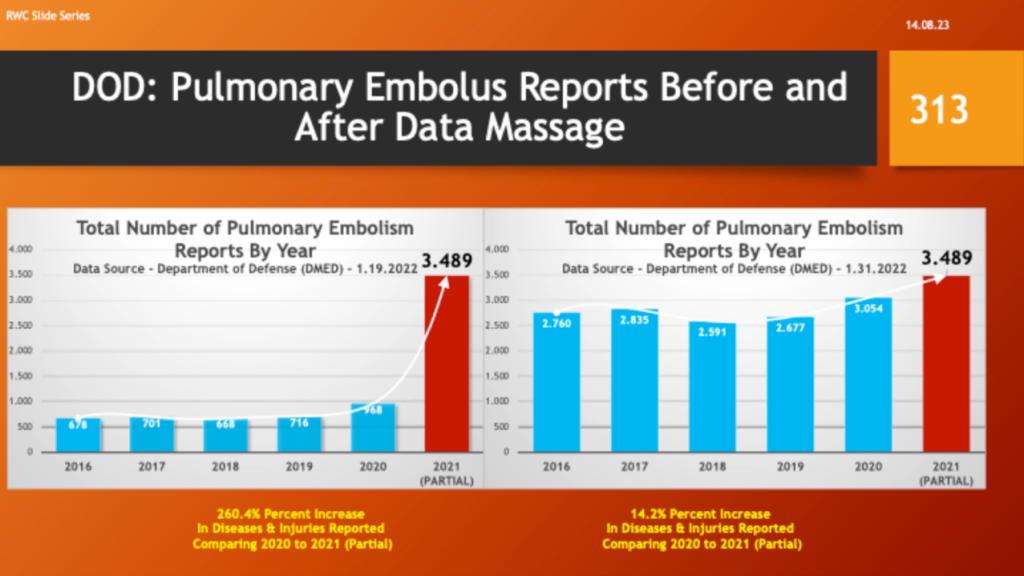
Pulmonary embolus reports went from an increase of 260% to a 14% increase.
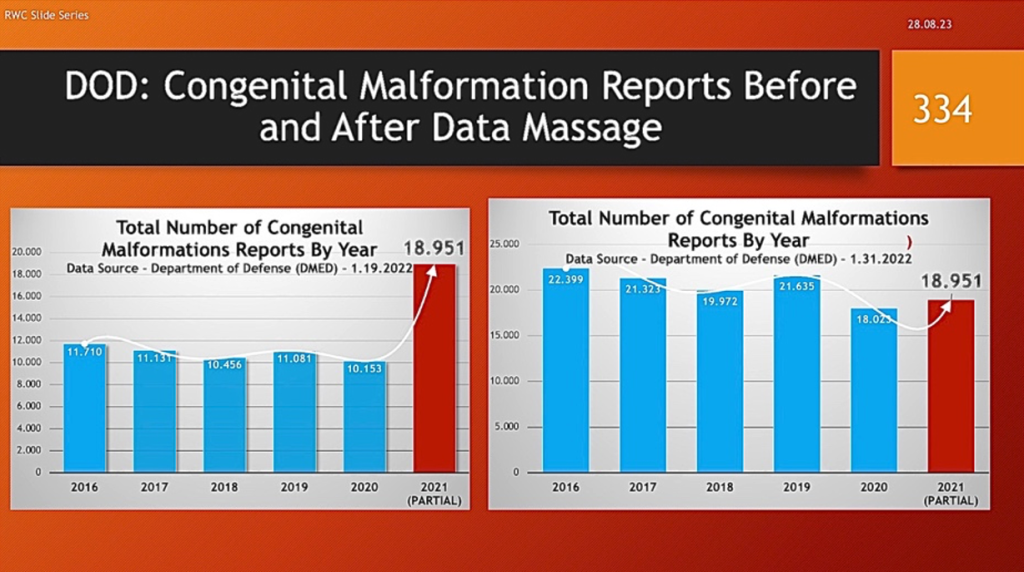
Congenital malformations went from a 174% increase to a 9% decline from the five-year average. The numbers on the left are very close to those that got thalidomide taken off the market.
“Nearly 60 years ago thalidomide was prescribed to treat morning sickness in pregnant women. What followed was the biggest man-made medical disaster ever, where over 10,000 children were born with a range of severe and debilitating malformations. Vargesson N. Thalidomide-induced teratogenesis: history and mechanisms.” [Birth Defects Res C Embryo Today. 2015 Jun;105(2):140-56. doi: 10.1002/bdrc.21096. Epub 2015 Jun 4. PMID: 26043938; PMCID: PMC4737249.]
Attorney Tom Renz reviews this glitch/data massage with information he obtained from three military whistleblowers beginning at approximately four hours, 52 minutes during Senator Ron Johnson’s hearing in January 2022. The entire five-hour recording is worthwhile.
To his credit Senator Ron Johnson has continued to pursue the matter but without success,
For over a year, Sen. Johnson has been examining DMED data integrity issues. His oversight work in this area began in late Jan. 2022, when he received information from three DoD whistleblowers showing significant increases in certain registered diagnoses in DMED in 2021 compared to a five-year average from 2016-2020. Since then, the senator has sent DoD and its contractor, Unissant Inc., multiple letters seeking information about the data published in DMED.
Sen. Johnson wrote to Sec. Austin, “I am grateful to the whistleblowers who continue to come forward to provide my office with information you and other DoD officials are unwilling to produce.”
Previous letters regarding the senator’s oversight of DMED data integrity issues can be found below:
March 7, 2022, to Unissant, Inc.,
June 14, 2022, to Unissant Inc., and
Forensic examination of the DMED data file is required to determine whether it has been tampered with. It the meantime, DMED data cannot be trusted.
3. Evidence of VAERS Data “Massage.”
Albert Benavides is a specialist in medical billing and forensic analysis of this complex and Byzantine system. He compiled the list below to summarize his findings of some of the “irregularities” in the VAERS data. He has the evidence. [https://www.vaersaware.com/my-powerpoint]

Mr. Benavides calls this technique “throttling.” Reports are held back and then filed late. This strategy is odd since the data shows up later and documents the “massage.” Wouldn’t it be much easier just to tell the truth?

This process was independently observed and reported in War Room/DailyClout Report 75 in July of this year. A dose-related response to the spike protein-generating drugs was identified in the preclinical animal studies reported in Pfizer confidential document 2.4 Nonclinical Overview and the clinical trials. The black line in the graph below shows rapid take-off of adverse event reports as the number of doses increased, yellow line, in December 2020 and January 2021.
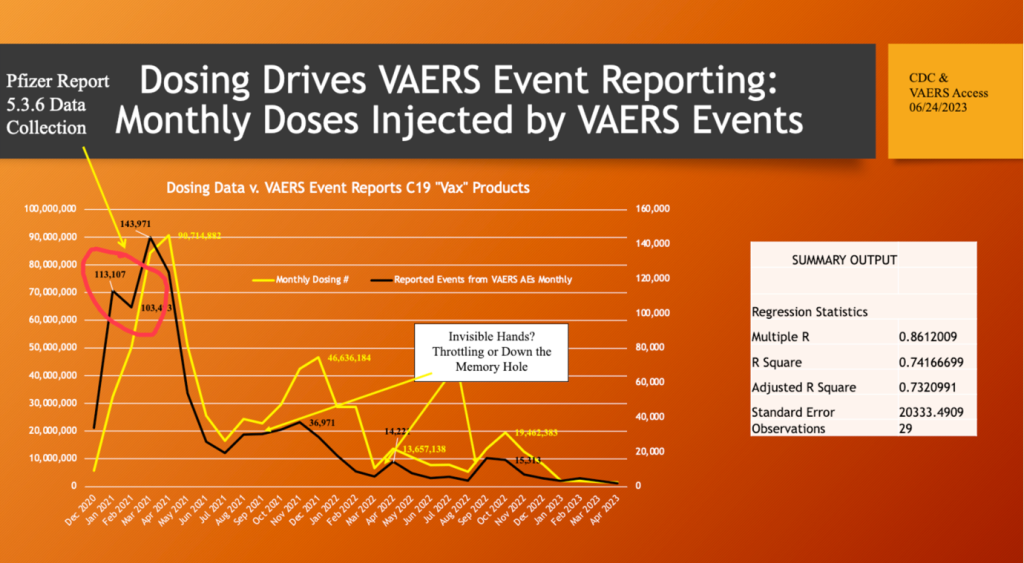
In February, the VAERS reporting tanked as the dosing continued up. This is an anomaly, and a number of factors could be involved here such as a change in the formulation, throttling as documented by Mr. Benavides, or something else.
There were three subsequent pulses of “vaccination.” There was a vastly reduced response in the VAERS reports relative to dosing levels compared with the initial pulse. This muted response in term of quantity of adverse event reports raises the same issues of formulation change versus data manipulation. Demographics may also play a role as the dosing program was age-based during 2021.
In addition to the anomalies listed by Mr. Benavides, another anomaly concerns the distribution of adverse events between females and males. Special toxicity of LNP/mRNA products in women was documented in War Room/DailyClout Report 38, “Women Have Two and a Half Times Higher Risk of Adverse Events Than Men. Risk to Female Reproductive Functions Is Higher Still.”
The ratio between females and males in VAERS was steady with approximately 60% of adverse events reported in females for 30 years. In December 2020, this ratio turned even more lopsided with more than 76% of adverse event reports reported in females with the launch of the BNT162b2-inoculation program.
The plot below shows that the percentage of female adverse event reports in VAERS gradually dropped to historic levels of ~60% in late 2021, and then held steady around 60% for the full year of 2022.
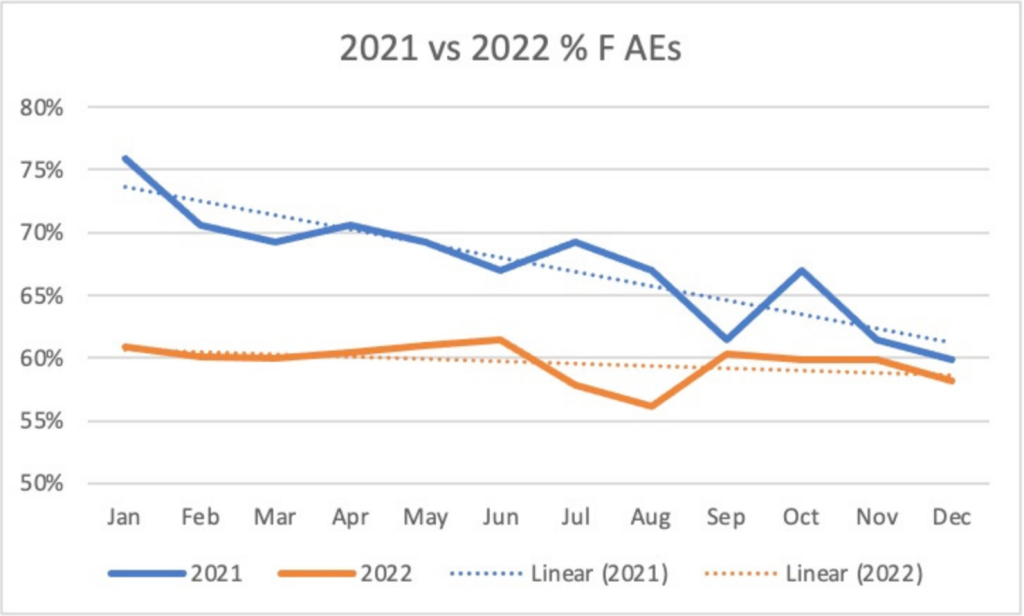
Yet, in the Pfizer documents (Confidential Document 5.3.6, Appendix 2.1, Appendix 2.2), women continued to exceed historic levels with 72% or greater of the adverse events. The Pfizer documents were collected concurrently with VAERS and now have millions of reports.
Was this data manipulation (“normalization”) or manipulation of the LNP/mRNA formulation or both? The VAERS data is for all COVID-19 gene therapy products.
The same trend is present with only the BNT162b2 product data shown below. The female predominance averaged 67% for the first 13 months then dropped to 59% for the second 12 months.
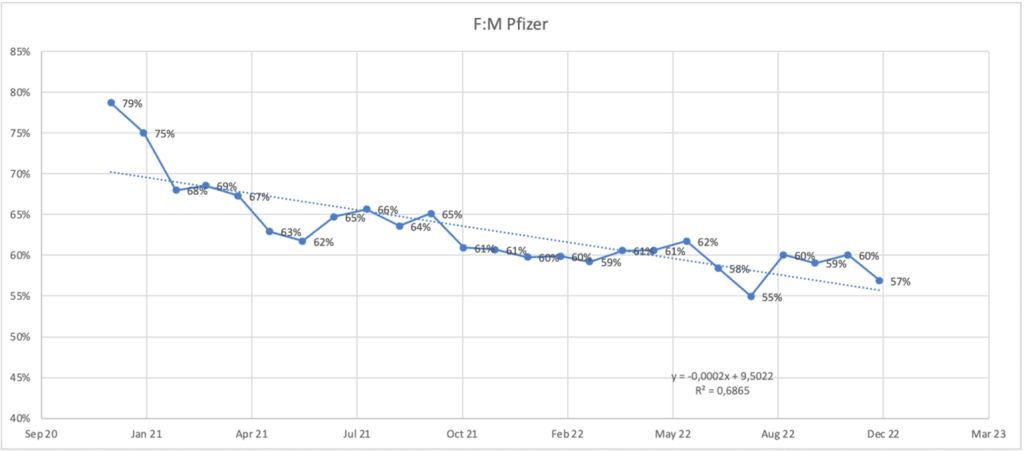
Robert F. Kennedy, Jr. was criticized for correctly citing research that showed unequal distribution of adverse events across different demographics, intentional or not.
Here we see a substantial difference between females and males, far above the 30-year baseline of ~60%. Is this phenomenon related to dose, XX targeting, or data manipulation? The second possibility is very disturbing.
Let us hope the Rhesus macaque non-human primates got loose from their lab cages at night and monkeyed with the data rather than the formulation of the LNP/mRNA products.
We need honest surveillance systems.

One of our country’s most important freedoms is that of free speech.
Agree with this essay? Disagree? Join the debate by writing to DailyClout HERE.



