Career scientists are struggling with attention to detail in fulfilling their vaccine safety duties. Their conclusions, marked by errors and oversights, have led to lives lost and debilitating disorders, as proven by the government scientists’ own review documents. In spring 2021, the US Food and Drug Administration (FDA) granted emergency […]
“Were We Lied To By the FDA?”
In this embroidery, a magician/judge swirls his robe around. It is not clear whether ‘Comirnaty’ or ‘Pfizer’ will appear on the robe. It is a magician’s trick. Behind the magician/judge, there are many shadows. They are distorted and scary. I was inspired by Stevan Douglas Looney’s Report 12: “Were […]
Report 94: Pfizer Secretly Studied a Heart Damage Marker, Troponin I, in Five- to 15-Year-Olds, Following mRNA COVID Vaccination in 2021.
We warned that there was proof that the Pfizer BNT162b2 mRNA COVID vaccine caused heart damage in teens and young adults, as early as May of 2021. As more information surfaces from the court-mandated release of Pfizer clinical trial documents by the FDA, and via FOIA’d emails, the CDC’s cover-up […]
Amid Revelations of Contamination, DailyClout Finds: FDA Withheld 16,000+ Pages of Quality Control Documentation for Pfizer mRNA Vaccine, Citing ‘Trade Secrets.’
Multiple independent labs have confirmed that Pfizer’s COVID vaccine is contaminated with DNA fragments, SV40, and plasmids. Given that, surely it is unethical for the Food and Drug Administration (FDA) to withhold the Pfizer vaccine quality data from Americans. Obviously, all eyes are now turning to Pfizer’s manufacturing process. What […]
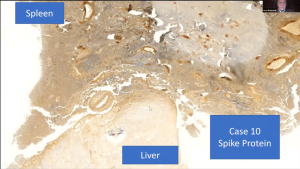
Report 85: “The Underlying Pathology of Spike Protein Biodistribution in People That Died Post COVID-19 Vaccination” – Dr. Arne Burkhardt
The Scientific and Medical Advisory Committee hosted a meeting with special guest speaker Professor Arne Burkhardt. Dr. Arne Burkhardt Compiled and edited by Robert W. Chandler MD MBA* *A transcript was produced then edited with a diligent effort to leave the meaning unchanged. My additions are in italics. […]
Pfizer’s Comirnaty mRNA COVID Vaccine Manufacturing Has Dropped by 89% Due to Decrease in Demand
The Follow the Money Team’s new report on Pfizer’s financials, based on Pfizer’s Q1 2023 reporting, offers many important insights: Pfizer reports that its mRNA COVID vaccine, Comirnaty®, manufacturing activities, performed on behalf of BioNTech, dropped 89% to only $5 million quarter-over-quarter. In its Q1 2023 report, Pfizer assumes no […]
“Where’s my cycle?” v. European Commission: Tens of Thousands of European Women Demand Answers Regarding Undesirable Menstrual Effects Following COVID-19 Vaccination.
Attorney Diane Protat is “counsel for the collective “Where is my cycle?,” which groups together tens of thousands of women in Europe who suffer from the undesirable effects of the Covid 19 vaccination on their menstrual cycle: amenorrhea, menorrhagia, adenomyosis, endometriosis, polycystic ovarian syndrome, miscarriage or even hysterectomy.” In October […]
Report 63: In Q3 2022, Pfizer Wrote Off $450 Million of Expired or Expiring COVID-19-Related Inventory.
Despite generating over $100 billion in revenue in 2022, Pfizer’s stock has dropped precipitously. Pfizer’s revenue was relatively flat, hovering between $41 billion and $51 billion, from 2014 to 2020. Then, its mRNA COVID-19 “vaccine” was awarded Emergency Use Authorization (EUA) by the Food and Drug Administration (FDA) on December […]
Report 56: Histopathology Series Part 1 – Autopsies Reveal Medical Atrocities of Genetic Therapies Being Used Against a Respiratory Virus
Summary: Dr. Arne Burkhardt is one of eight international pathologists, physicians and scientists who were asked to perform a second autopsy, requested by friends and family of the deceased who were not satisfied with the results of the first autopsy. Thirty autopsies and three biopsies were evaluated; 15 cases […]
Letter to the FDA: COVID-19 ‘Vaccines’ and COVID-19 Infection Linked to Myocarditis – What Do They Have in Common?
Peter Marks, MD, PhD Director, Center for Biologics Evaluation and Research U.S. Food and Drug Administration 10903 New Hampshire Avenue WO71-7232 Silver Spring, MD 20993 Peter.Marks@fda.hhs.gov December 18, 2022 Dear Dr. Marks, I am troubled by the following, and look forward to your studied response on my single […]
Report 39: Despite Incomplete Safety Trials, the Food and Drug Administration (FDA) Grants Full Approval to Pfizer-BioNTech’s COMIRNATY® for Adolescents 12-15 Years of Age
Without a completed safety study or expert committee review, the FDA issued a supplemental Biologics License Application (“sBLA”) approval letter granting full FDA approval to Pfizer-BioNTech’s COMIRNATY® COVID-19 mRNA vaccine for use in children ages 12-15. This was done even though safety study completion, on which approval should be based, […]
The Food and Drug Administration (FDA) Grants Full Approval to Pfizer-BioNTech’s COMIRNATY for Adolescents 12-15 Years of Age Despite Incomplete Safety Trials
Without having assembled an expert committee, the FDA issued a supplemental BLA (“sBLA”) approval letter granting full FDA approval to Pfizer-BioNTech’s COMIRNATY COVID-19 mRNA vaccine for use in children ages 12-15. They did this despite the fact that the safety study on which approval should be based will not be […]
Report 31: Pfizer-BioNTech “Equivalent” Half Truths or a “Lot” of Lies?
Pfizer-BioNTech FDA-Approved “Equivalent” Half Truths or a “Lot” of Lies? This Report was written exclusively for DailyClout by the Members of the Pfizer Analysis team. It should not be copied or republished without permission from DailyClout or a full credit and link toDailyClout.io Summary The public was not told […]
Report 14: Were We Lied to by the FDA?
What’s the difference between Pfizer’s FDA approved COMIRNATY and the emergency use authorized “vaccine?” Only the law, not science, says the FDA. Were we lied to by the U.S. Food and Drug Administration (FDA) and the media when they told us that, if we received the Pfizer “vaccine” after […]