A Drug Safety Physician’s Correspondence with the FDA: A Quest for Accuracy in Investigational Bivalent mRNA COVID-19 ‘Vaccine’ Adverse Event Reporting
Introduction
On November 4, 2022, the Centers for Disease Control and Prevention (CDC) and U.S. Food and Drug Administration (FDA) concluded that, based on Vaccine Adverse Events Reporting System (VAERS) and v-safe data received between August 31, 2022, and October 23, 2022, “Health care providers and patients can be reassured that adverse events reported after a bivalent booster dose [COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5)] are consistent with those reported after monovalent doses.” (https://www.cdc.gov/mmwr/volumes/71/wr/mm7144a3.htm?s_cid=mm7144a3_w)
Unlike the flu vaccine you may receive this fall, the bivalent COVID-19 ‘vaccines’ are:
- Unapproved, investigational products still under Emergency Use Authorization (EUA) only
- Currently being tested in clinical (human) trials
- Authorized based on “the totality of the scientific evidence available:” zero data from the actual vaccine product in human
(https://www.fda.gov/media/150386/download
https://www.fda.gov/media/144636/download
https://twitter.com/DrCaliff_FDA/status/1562837777905233921)
In my professional medical opinion, the bivalent ‘vaccines’ should never have been released to the public at their stage of development. Also, I consider passive surveillance systems such as VAERS and v-safe to be vastly inappropriate as standalone safety evaluations for these investigational biologics. But, in an attempt to help make the evolving safety profiles of the bivalent ‘vaccines’ more accurate, transparent and evaluable, I reached out to the FDA with my concerns regarding adverse event reporting.
My letter below summarizes the current status of my requests of the FDA, with an additional request for a status update.
The FDA responded back to me on November 3, 2022: “We will review the letter and respond when we are able.” My hope is that they reanalyze their “early safety findings” (August 31, 2022, to October 23, 2022) in light of the safety concerns I raised. (https://www.cdc.gov/mmwr/volumes/71/wr/mm7144a3.htm?s_cid=mm7144a3_w)
Letter to the FDA
November 2, 2022
Dear Dr. Marks,
Below is a table reflecting the current status of my recent requests of the FDA regarding accurate and comprehensive reporting of adverse events to the Vaccine Adverse Event Reporting System (VAERS) in association with the investigational bivalent Covid-19 ‘vaccines.’ I appreciate the FDA’s action taken on two of four of the items. I would greatly appreciate your response as to the status of the remaining two items.
| My Request to FDA | Date of My Request | FDA Response Back to Me (date) | Status |
| “On one of the manufacturer’s websites, Pfizer’s in this case, directed by the EUA Fact Sheet for reporting, there is NO drop-down or specification for the bivalent vaccine product — the closest match is a static “COVID 19” vaccine option. Also, there is NO instruction to the Reporter to even write in the bivalent vaccine suspect product in any field, such as adverse event description”
https://covaes.pfizersafetyreporting.com/en
|
09SEP2022 | “We will review and get back to you when we’re able.” (12SEP2022)
“For the concern about the Pfizer Safety Reporting Website, we have contacted the manufacturer to address this issue.” (15SEP2022) |
Done (bivalent checkbox added to Pfizer’s online AE reporting form). |
| “In the online VAERS reporting instructions [Item 17], the Reporter is asked to “Select a U.S.-licensed vaccine if applicable; otherwise scroll down…and select “Other”:
Despite the bivalent drop-downs, this might present a conflict to some of the more conscientious reporters, resulting in “Other” being chosen over the bivalent option—data would be lost. It is also misleading in that it implies the bivalent vaccine is licensed.” https://vaers.hhs.gov/esub/index.jsp
|
09SEP2022 | “We will review and get back to you when we’re able.” (12SEP2022)
“…we are working with our colleagues at CDC to modify the VAERS instructions to clarify that individuals should chose the specific vaccine name whether it be fully licensed or available under Emergency Use Authorization. “ (15SEP2022) |
Done. Item 17 now reads: “Select a U.S.-licensed or authorized vaccine if applicable…”
|
| “The VAERS online reporting form does not capture all prior covid-19 vaccine injections in the series (primary or booster) since the December 2020 deployment: only “Date of vaccination [Item 4]” and “Any other vaccines received within one month prior to the date of vaccination [Item 22]” are captured.”
“There is no way to collect product information to detect cumulative toxicity data from exposure of the various covid-19 vaccines since initiation in December 2020. Adverse events found to be dose-dependent and/or temporally related (such as myocarditis, vaccine-induced-immune thrombotic thrombocytopenia, etc…), may only be observed with a complete vaccination history (beyond one month).” “As a solution, VAERS form “Item 22” might be edited to also include all prior covid-19 vaccinations, not just those within one month of item 4 (vaccination date associated with currently reported AE). Although the VAERS form is intended to be generic across all vaccines, due to the unique investigational phase of some of the covid-19 vaccines, as well as the requirement for ongoing safety studies even for the “original” products, it is imperative to immediately modify the VAERS form to include a special section, or special instructions, to capture vaccination history for a recipient’s entire covid-19 vaccine series.” https://vaers.hhs.gov/esub/EsubController |
19SEP2022 | “We appreciate your comments and suggestions below, and have shared with the appropriate staff for consideration.” (21SEP2022) | I don’t see evidence that this has been addressed. |
| “I have checked VAERS for the month of September to see if suspect bivalent Covid-19 vaccines were erroneously being reported [to VAERS by Moderna or Pfizer] under the monovalent (‘original’) “Vaccine Product” category.” “I found [… ] reports with “bivalent” in the AE Description that were categorized under the ‘original’ monovalent vaccine.” “[I]t’s critical to accurately capture the safety profile of the bivalent vaccine as compared to the ‘original’ monovalent vaccine.” | 02OCT2022 | “Thank you for your message. We will look into this further.” (02OCT2022) | I don’t see evidence that this has been addressed. As seen with the examples below, this appears to still be occurring.* |
*VAERS ID: 2481257-1:
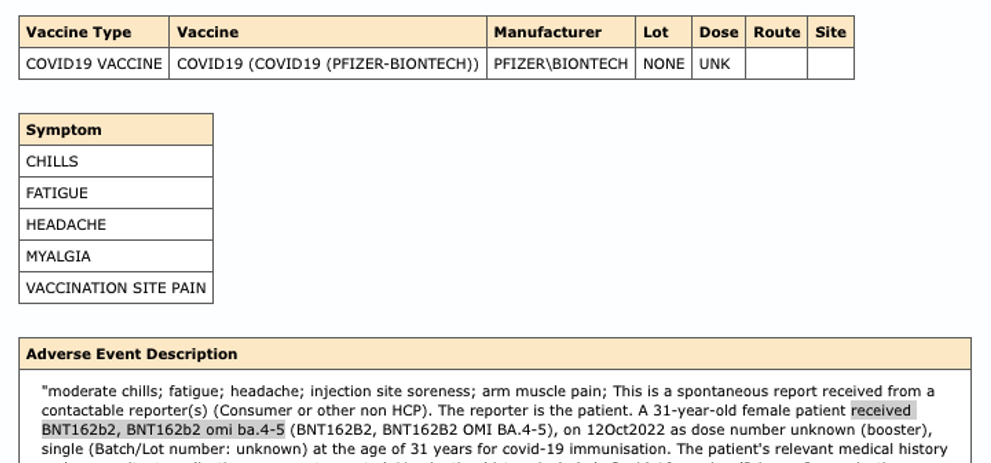
*VAERS ID: 2481090-1:
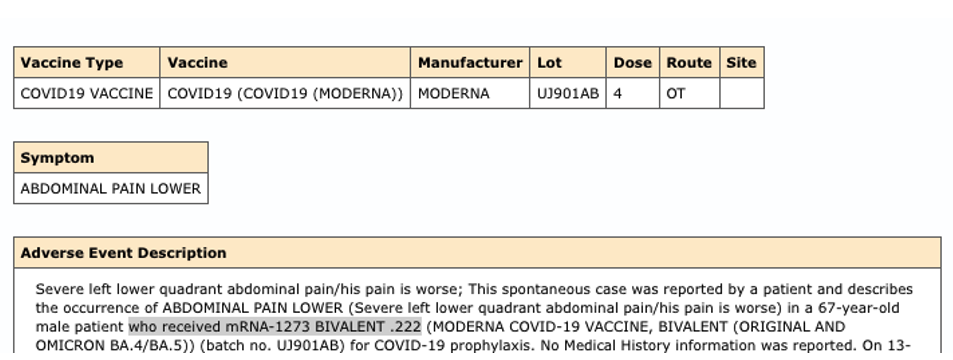
Sincerely,
Carol Taccetta, MD, FCAP
Please note: the views expressed here are Dr. Taccetta’s personal opinions and do not reflect the views of former or current employers or any professional organizations to which she belongs.
One of our country’s most important freedoms is that of free speech.
Agree with this essay? Disagree? Join the debate by writing to DailyClout HERE.





Consistently made over $26,000 in greater profits from domestic with the gain of easy playback and sticky on line interest. ~”Z70 I in reality made $18,636 with this best domestic profits. Everyone can now with out a doubt. …
make extra cash on line through using—— http://works360.blogspot.com
Someone in charge of designing a VAERS or other reporting systen will claim that their system appears amateurish due to internal miscommunication or something similar but equally innocent. I clearly see that the confusion in tbs reporting system is by design and that’s evil!
Someone in charge of designing a VAERS or other reporting systen will claim that their system appears amateurish due to internal miscommunication or something similar but equally innocent. I clearly see that the confusion in the reporting system is by design and that’s evil!