Report 84: War Room/DailyClout Research Team Breaks Huge Story: More Cardiovascular Deaths in Vaxxed Than Unvaxxed; Pfizer Did Not Report Adverse Event Signal; Death Reporting Delays Favored Pfizer/Vaccinated.

War Room/DailyClout Pfizer Documents Analysis team members Corinne Michels, PhD; Daniel Perrier; Jeyanthi Kunadhasan, MD; Ed Clark, MSE; Joseph Gehrett, MD; Barbara Gehrett, MD; Kim Kwiatek, MD; Sarah Adams; Robert Chandler, MD; Leah Stagno; Tony Damian; Erika Delph; and Chris Flowers, MD have published a bombshell study titled, “Forensic Analysis of the 38 Subject Deaths in the 6-Month Interim Report of the Pfizer/BioNTech BNT162b2 mRNA Vaccine Clinical Trial.”
Highlights from the Study:
- This is the first study of the original data from the Pfizer/BioNTech BNT162b2 vaccine clinical trial to be carried out by a group unaffiliated with the trial sponsor.
- Thirty-eight (38) trial subjects died between July 27, 2020, the start of Pfizer’s Phase 2/3 of its clinical trial, and March 13, 2021, the data end-date of Pfizer/BioNTech’s Six-Month Interim Report.
- At Week 20 of the trial, Pfizer’s BNT162b2 mRNA vaccine received Emergency Use Authorization from the FDA, and subjects in the placebo arm were given the option to get vaccinated. Most accepted.
- For the first 20 weeks of trial, before the placebo cohort began receiving vaccines, there was no significant difference in the number of deaths in the vaccinated versus placebo arms of the trial.
- Once the placebo cohort was unblinded and began receiving the Pfizer’s vaccine, following Week 20, deaths among the vaccinated subjects continued at the same rate, while deaths among the unvaccinated slowed and even plateaued.
- For the first 20 weeks of trial, before the placebo cohort began receiving vaccines, there was no significant difference in the number of deaths in the vaccinated versus placebo arms of the trial.
- Evidence found of an over 3.7-fold increase in number of deaths due to cardiovascular events in vaccinated subjects compared to placebo subjects. This significant adverse event signal was not reported by Pfizer/BioNTech. (In other words, Pfizer knew about this safety signal by 3/13/21 and hid it.)
- Three hundred and ninety-five (395) subjects were “lost to follow-up.”
- Patterns of delay:
- ” Of the 8 [Pfizer] BNT162b2 vaccinated subjects that should have been reported to the VRBPAC [Vaccines and Related Biological Products Advisory Committee] on December 10, [2021], the median reporting delay was 18 days (average of 17.5 days). Among the 8 Placebo subjects, the median delay was 5 days (average of 5.9 days) “
- “When the recording delay after December 11 is analyzed, we found a dramatic decrease in both arms of the trial. The median delay in the BNT162b2 arm of the trial was 7 days (average 9.8 days) and in the Placebo arm the delay was 3 days (average of 15.9 days).”
- ” These results are a clear demonstration that the long official recording delays are not distributed equally between the two arms of the trial but are clustered in the BNT162b2 vaccinated arm, particularly before FDA [Food and Drug Administration] approval of the EUA [Emergency Use Authorization]. Once the EUA was approved, Pfizer/BioNTech reported the date of death in a timelier fashion, although delays were still longer among vaccinated subjects.” [Emphasis added.]
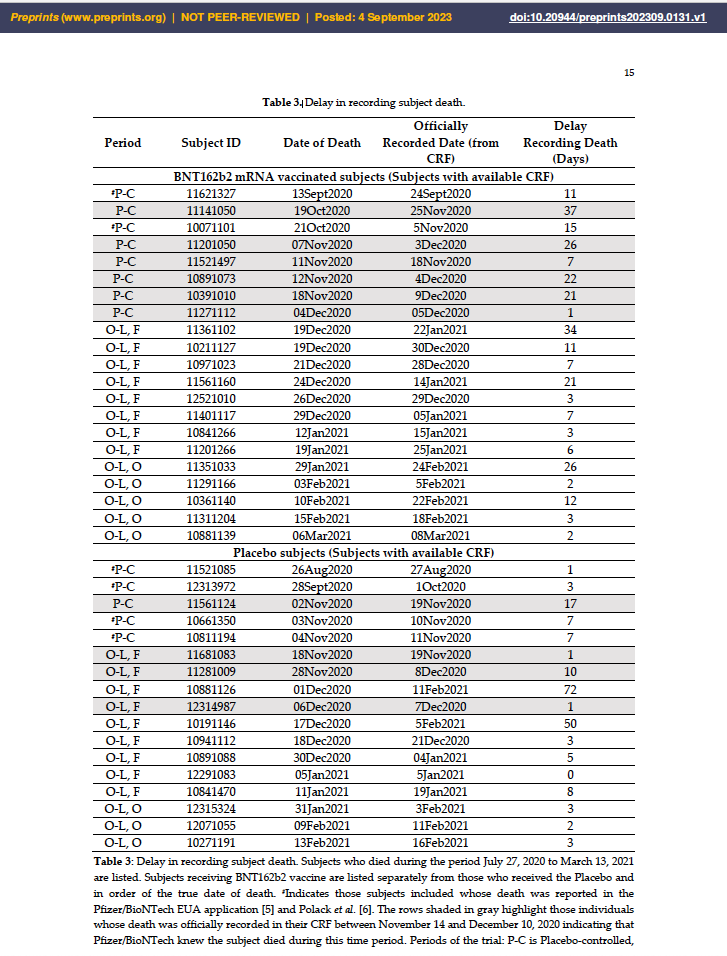
- “Our analysis of the data in Table 3 [below]…showed that Pfizer/BioNTech used the date that the death was officially recorded in the CRF to determine which time period to report the death NOT the actual date of death, although both were available to them.” [Emphasis added.]
- According to the CA4591001 Protocol, Pfizer/BioNTech was to be notified of a subject death immediately.
- Access is not available to records that would have confirmed that the trial sites were diligent regarding death notifications; however, the existence of other steps in the death notification process are alluded to in the Case Report Forms [CRFs], which could have played a role in delaying entries into the CRF.
- Preliminary databases, such as a Death Details Form, are suggested in interactions logged into the CRFs.
- Public access is not available to any of these.
- Completion of the Death Details Form, and perhaps other requirements, appears to be partly computerized and automatic.”
- Additional Commentary:
- As was also shown in the timing of test results in War Room/DailyClout Report 76 around the United States’ 2020 presidential election, there appears to be a consistent pattern of delay which always favors Pfizer’s interests. A release of or subpoena for the records in the “Death Details Form” and other related data would be required to show that the timing delays were not intentional.
Explainer Video Produced by Chris Flowers, MD:
Please read this important study by going to either of the links below or using the embedded PDF.
https://www.preprints.org/manuscript/202309.0131/v1
[https://doi.org/10.20944/preprints202309.0131.v1]





@DrNaomiRWolf
@Raheem
@stevebannon
#maga
A group of us in the WR Posse refuse to let the left get away with the false narrative of using the descriptor, “the vaccine hesitant”. We are now to be referred to as the “Nuremberg Movement”.
#maga
#americafirst
#nuremburgmovement
I have written an essay on the propagandistic nature of the “Vaccine Hesitant” label.
http://dcwritings.com/wp-content/uploads/VaccineHesitancy.pdf