United Kingdom Health Agency Gets FOIA Requesting All AstraZeneca COVID-19 Vaccine Data
A legally-produced, effective Freedom of Information Act (FOIA) request was sent to Dame June Raine, Chief Executive of Medicines and Healthcare Products Regulatory Agency (MHRA), on behalf of the Health Advisory and Recovery Team (HART), requesting that the MHRA fully produce all the data, information and documents submitted to them by AstraZeneca that underlies the Conditional Marketing Authorisation (Temporary License) of the AstraZeneca COVID-19 vaccine (a non-replicating viral vaccine also known as Vexzevria and Covishield).
FOI_AZ Dame Raine MHRAWHY IS AN FOI REQUEST OF THE MHRA TO RELEASE ALL ASTRAZENECA DATA NEEDED?
Why bother, and what do we hope to achieve?
Lawyers from PJHLaw submitted an FOI request on behalf of HART – a group of doctors, scientists, economists, psychologists and other academic experts – to the MHRA, requiring all data that was submitted by AstraZeneca in the application for license of their COVID- 19 vaccine (AZD1222/Vaxzevria) and relied upon in granting a Conditional Marketing Authorisation for its use. Specifically, the following was requested:
- Pre- and post-authorisation safety and efficacy data for this product.
- All information that allowed a “rigorous scientific assessment” of all the available evidence of quality, safety and effectiveness by the UK Regulator, the Medicines and Healthcare Products Regulatory Agency (MHRA).
- All information and full data set that the MHRA stated their expert scientists and clinicians reviewed from the laboratory preclinical studies, clinical trials, manufacturing and quality controls, product sampling and testing of the final vaccine and the conditions for its safe supply and distribution.
- Anonymised data from their clinical trials.
Why Was This Request Submitted?
Since March 2020, many have been questioning the scientific evidence for much of the COVID-19 pandemic management, in particular restrictions driven by a narrative of fear, the lack of any early treatment protocols and the rapid development of COVID “vaccines” rolled out to an entire population.
“Safe and Effective” was the marketing banner whenever the “vaccines” were being discussed by the MHRA, the mainstream media or Big Pharma. But at all ages, it is clear that properly informed consent has been set aside, in contravention of the General Medical Council Good Practice Guidelines.
Raw data surrounding all the COVID-19 vaccines and related contracts have been shrouded in mystery. There has also been a climate of secrecy. This is contrary to standard practice of drug research, where full publication in peer-reviewed journals should include access to anonymised raw data. Indeed, the capture of academia by Big Pharma threatens to undermine evidence-based medicine.
Meanwhile the overstating of efficacy and understating of harms continues unabated, and the UK efforts to persuade uptake have been matched by more serious coercion in many countries.
A review of the Association of British Pharmaceutical Industry (ABPI) Code of Practice governing sales and marketing within Pharma companies is revealing. It states:
Clause 6.1: Absolute risk and relative risk. Referring only to relative risk, especially with regard to risk reduction, can make a medicine appear more effective than it actually is. In order to assess the clinical impact of an outcome, the reader also needs to know the absolute risk involved. In that regard, relative risk should never be referred to without also referring to the absolute risk. Absolute risk can be referred to in isolation.
Yet, how were the vaccines marketed? – Entirely on Relative Risk Reduction. This is vitally important when it comes to vaccinating younger, healthy people, whose absolute risk from COVID-19 is orders of magnitude lower than for the frail and elderly.

Clause 6.4: It must not be stated that a product has no adverse reactions, toxic hazards or risks of addiction or dependency. The word SAFE must not be used without qualification.
Dr. Alison Cave (Chief Safety Officer, MHRA), states:
“Patient safety is our highest priority. The Covid-19 vaccines ……were approved after a rigorous review of the safety, quality and effectiveness of the vaccines by the MHRA and the government’s independent advisory body, the Commission on Human Medicines (CHM). The MHRA concluded that the Covid-19 vaccines were safe and effective, and the benefits of the vaccines outweigh any risks.”
She stated that the MHRA operates the Yellow Card system on behalf of the CHM. She also stated that an adverse drug reaction report (ADR) associated with a fatal outcome does not mean that the vaccine caused death. (How do they know that if they have not investigated the signal?)
On 19 March 2020, just before the first UK lockdown was announced, COVID-19 was no longer considered to be a high consequence infectious disease (HCID) in the UK, and the Advisory Committee on Dangerous Pathogens (ACDP) was also of the opinion that COVID-19 should no longer be classified as an HCID. Her Majesty’s Government (HMG) was given this information before it introduced lockdown.
Professor Neil Ferguson’s model on which he based the necessity for lockdown has been criticised worldwide. In March 2020, Ferguson predicted 510,000 would die from COVID-19 in the UK (2.2 million in US). The Imperial College model of the COVID-19 disease was severely criticised.
Bob Seely, Member of Parliament: “Never before has so much harm been done to so many, by so few, based on so little questionable, potentially flawed data”.
Richards & Boudnik of WANdisco, a global leader in Big Data software, said of the totally unreliable modelling that was used in many countries to bring in draconian lockdowns, “Neil Ferguson’s Imperial model could be the most devastating software mistake of all time, in terms of economic costs and lives lost.” (Telegraph, 16.05.2020, https://www.telegraph.co.uk/technology/2020/05/16/neil-fergusons-imperial-model-could-devastating-software-mistake/)
We started testing using the PCR test. The false positive rate (FPR) was 0.8% and, because of the numbers being tested, the chance of a positive case being false was 89-94%. This testing defined the “cases,” the number hospitalised and the number of deaths. Never had we previously tested the healthy to tell them they are positive for something for which they have no symptoms. If they did get infected, then 99.97% were fine, and 0.096% (Hansard recorded) could die; but those who died were an average age of 82.5 years (average life expectancy at 81.5 years). We also began quarantining healthy contact, another first.
The “vaccine” rollout was sold as the way out of the “pandemic” and the only way to get “herd immunity.” These three terms all had their definitions changed by prominent public health entities.
MHRA (Medicines and Healthcare Products Regulatory Agency) responsibilities:
- Ensuring that medicines meet applicable standards of safety, quality, and efficacy
- Helping to educate the public and healthcare professionals about the risks and benefits of medicines leading to safer and more effective use
- Supporting innovation and research and development that is beneficial to public health
- Influencing UK, EU, and international regulatory frameworks so that they are risk- proportionate and effective at protecting public health
The MHRA retains responsibility for pharmacovigilance (PV) across the UK. Pharmacovigilance is the continuous drug safety monitoring of medicinal drugs manufactured by pharmaceutical companies. The underlying objectives of PV:
- Prevent harm from Adverse Drug Reactions (ADRs) in humans arising from the use of authorised medicinal products.
- Promote the safe and effective use of medicinal products, through providing timely information about the safety of medicinal products to patients, Healthcare Professionals and the public.
How many knew their infection risk?
Infection Fatality Rate, (IFR) UK:
0-19 0.0027%
20-29 0.014%
30-39 0.031%
40-49 0.082%
50-59 0.275%
60-69 0.59%
70+ 2.4% (non-institutional)
70+ 5.5% (all))
(UKHSA)
Announcement by the Chief Executive of the MHRA, Dame June Raine, at the MHRA brief on the AstraZeneca vaccines:
- In this 30 December 2020 video, Dr. June Raine states that the AstraZeneca COVID-19 vaccine can be given to anyone 18 and over, in two doses 4-12 weeks apart (average weeks between doses in the trials were 3 weeks).
- Safety of the public comes first, and this comes after a thorough and scientifically rigorous review of all the evidence in terms of safety, effectiveness and quality.
- “We are facing one of the biggest threats to health, in UK and around the world.”
- Dr. Raine points out the vaccine “protects” against COVID-19, saving many thousands of lives. (Licenced to “protect” » safeguard against infection/transmission.)
- Chair of CHM, Professor Sir Munir Pirmohamed, states that the AstraZeneca COVID vaccines do not have an effect until Day 22, when one gets partial immunity, so continue with restrictions till then.
- There are no specific precautions if you have had COVID-19, and you do not need testing before the injection.
- Vaccines should be considered for pregnancy (and those breastfeeding) when the potential benefit outweighs the risks following individual talks with every woman and their HCP. (Medicines in pregnancy, breastfeeding and children usually takes many years of monitoring before use – why not these vaccines?)
- CHM advises that anyone with allergy to medicines and vaccines can have the Pfizer-BioNtech mRNA COVID-19 vaccine. Anyone with specific reactions to this vaccine or the ingredients, should not. (Even pharmacists do not know what the ingredients are.)
These are the conditions for authorisation for emergency supply under Regulation 174 for COVID-19 Vaccine AstraZeneca. This temporary authorisation under Regulation 174 permits the supply to and by the Crown of COVID-19 Vaccine AstraZeneca, based on the safety, quality and efficacy data submitted by AstraZeneca to MHRA in the period from 24/09/2020 to 29/12/2020.
Please note that a “robust” review of all the data was completed in three months.
It appears that, in the UK, 86% of the funding of the MHRA is provided by the Pharmaceutical Industry, the very companies whose products the MHRA regulate with the aim of protecting the public from harm. The MHRA are now intending to operate as an “Enabler” (here – 1:24hr) instead of a “Regulator” and, as such, intend to bring medicines to market within 100 days (The CEPI 100-day promise with justification of “safety”).
The MHRA is helping to create a self-appointed global regulatory body.
Dame June Raine stated, “Here in the UK, the MHRA worked really hard to overcome some of the obstacles within the structure of clinical trials taking place in different jurisdictions, and that was one of the reasons we were able to license vaccines here in the UK faster than anywhere else because of the flexibility yet robustness shown by the MHRA.” (For a review see here.)
We need to know that these first examples of a new technology are in fact safe and effective, that their data has been robustly reviewed as stated, and that clinical use has confirmed this.
The AstraZeneca type of vaccine uses an unrelated, harmless virus (the viral vector) to deliver SARS-CoV-2 genetic material. When administered, human cells use the genetic material to produce a specific viral protein, which is recognised by the immune system and triggers a response. This response builds immune memory, so one’s body can fight off the virus in future. Other preparations in use in UK, namely Pfizer and Moderna, are mRNA-based COVID-19 vaccine products. Similar principles apply – that a small section of the genetic code for the spike protein is injected into the recipient, where it must then cross cell membranes and instruct the cells to make their own spike protein against which one learns to produce antibodies.
There are two glaringly obvious problems with this. Firstly, the vaccines code specifically for the spike protein, which appears to be the most toxic part of the virus and only represents 5-10% of the whole. Thus, vaccine immunity could be predicted to be less effective against the inevitable future variants. Second, and perhaps more importantly, because of the designation of ‘vaccine,’ these products were not required to undergo full pharmacokinetic and biodistribution studies in humans or intergenerational reproductive studies in animals. It is not known how spike protein production varies between individuals, whether in terms of quantity, distribution or duration.
The AstraZeneca product remains in clinical trials until 2023. How much information these will provide is unclear, since it is known that trial participants were offered unblinding and to receive the vaccine within the first few months of the trial. The public needs to see long-term safety data.
A recap confirming what has happened took place in March 2022 at Sommerville College, Oxford, by Dames Bingham and Raine and can be seen here. It is a remarkable account of the workings of the Vaccine Task Force (VTF) that was set up to help the UK fight the SARS-Cov-2 outbreak and get the licensing of the vaccines. One may also detect a healthy dose of self-congratulation all-round. This is an analysis by Hedley Rees who is a UK-based consultant specialising in operations and supply chain management within life science, of the two presentations.
RATIONALE FOR THE FOIA REQUEST
The US Experience: Public Health and Medical Professionals for Transparency (PHMPT) is an organization in the United States (US) made up of public health professionals, medical professionals, scientists, and journalists. PHMPT exists for the sole purpose of disseminating to the public the data and information in the biological product files for each of the COVID-19 vaccines. In furtherance of its mission, and to ensure that the Food and Drug Administration (FDA), the US regulator of medicines, acts in furtherance of its commitment to transparency, PHMPT sought via a FOIA request to obtain the data and information relied upon by the FDA to license the Pfizer mRNA COVID-19 vaccine.
On September 9, 2021, the FDA denied PHMPT’s request for expedited processing on the basis that PHMPT did “not demonstrate a compelling need that involves an imminent threat to the life or physical safety of an individual” or “that there exists an urgency to inform the public concerning actual or alleged Federal Government activity.”
This FOIA request was eventually granted by court order through the PHMPT’s attorneys Siri & Glimstad LLP, (acting in behalf of Informed Consent Action Network (ICAN)). The judge required the FDA to release all 450,000 pages of information over eight months, despite the FDA planning to retain the data for 75 years. This is now being analysed by approximately 3,500 expert and 350 attorney volunteers. Evidence of fraud would, if found, negate any indemnity for Pfizer.
The judge in the FDA Pfizer court order trial used the following quotes:
“Knowledge will forever govern ignorance.” – James Madison
“A nation that is afraid to let its people judge the truth and falsehood in an open market is a nation that is afraid of the people.” – John F Kennedy
“Excessive administrative secrecy … feeds conspiracy theories and reduces the public’s confidence in the government.” – John McCain
More recently, the same legal team in the US have forced access to anonymised V-Safe registry data and have made it fully publicly accessible.
We are now doing the same in the UK.
A previous FOI request to the MHRA for its internal document specifying how its staff follow up individual Yellow Card reports for adverse events from medicines, resulted in this reply: “The MHRA does not hold a process for investigation of individual Yellow Card reports.” Look at the number of recorded reactions. Why are deaths not being investigated when the numbers are high?
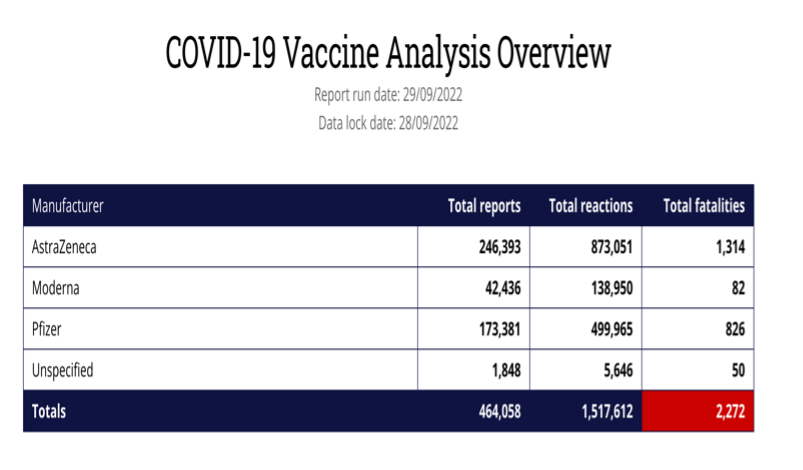
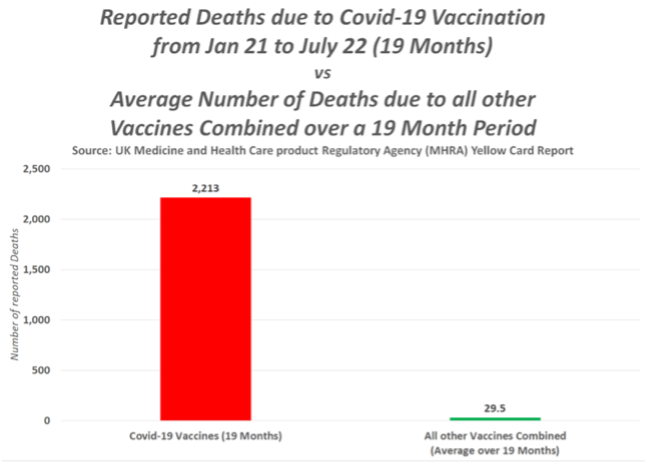
This is of paramount importance in the UK, as more than 58% of the vaccine ADRs and fatalities have occurred after the AstraZeneca vaccine according to the MHRA’s own Yellow Card reporting system. There are concerns because the Yellow Card reports which were published by MHRA on a weekly basis, have now moved to a monthly report and there has been no report since 24 August 2022. The AstraZeneca vaccine was licensed 30th Dec 2020 and first used on 4 January 2021 and was widely used in the UK in the first year of vaccination, though it appears to have been mysteriously dropped from the current booster programme. In the first few months, this included younger care workers and National Health Service (NHS) staff, many in the age range for which AstraZeneca was subsequently dropped.
Historically we know that:
1976: Swine flu (H1N1) pandemic developed.
- At 25 (eventually 53) deaths, it was stopped.
2009: Influenza A (H1N1) spread worldwide.
- Pandemrix by GlaxoSmithKline had 47 fatal reactions; it was removed from the market.
In 2018, Professor Peter Doshi, British Medical Journal, “Pandemrix was removed from the market after the pandemic, but the number of reported events indicate a need for a stronger screening process for pandemic vaccinations in the future.”
“The events of 2009-10 raise fundamental questions about the transparency of information. When do public health officials have a duty to warn the public over possible harms of vaccines detected through pharmacovigilance? How much detail should the public be provided with, who should provide it, and should the provision of such information be proactive or passive?”
“If history were to repeat itself, does the public have a right to know?”
Yellow Card reports are estimated to represent <10% of the actual adverse events. The number of deaths from the AstraZeneca COVID-19 vaccine is now 45 times the level when a drug would normally be pulled. Does the MHRA have a defined point at which it pulls a drug or vaccine and, if not, why not?
Governments have told the world and our children that the COVID-19 genetic vaccines are as safe as other vaccines while bearing the evidence of unprecedented harms, including death and debilitating injuries, in their own pharmacovigilance databases.
In Britain, 2,407 people had applied for vaccine injury compensation by August 2022. By paying out from the Vaccine Injury Compensation Program to 12 vaccine injury victims, HMG have now acknowledged that death and serious injury has been caused by COVID-19 vaccination.
Coroners’ Courts are also registering deaths because of COVID-19 vaccination. AstraZeneca knows deaths have been caused by the vaccine due to Coroners’ Inquests. Kelly Hatfield, daughter of Ken Purnell who died from the AstraZeneca vaccine, interviewed on Mark Steyn – GB News and confirmed that the Leicester Coroner’s Court Inquest was a documented inquest and involved the reading of the report and post-mortem. No one was allowed to speak, but AstraZeneca lawyers and HMG lawyers “attended” via Microsoft Teams. Neither have reached out to Ms. Hatfield’s family, and this fatal ADR has never been followed up on.
Sir Christopher Chope is introducing a COVID-19 Vaccine Damages Bill to require the Secretary of State to establish an independent review of disablement caused by COVID-19 vaccinations and the adequacy of compensation offered to persons so disabled and for connected purposes.
It needs to be recognised that these patterns of ADRs resulting from the COVID-19 vaccines are being seen all over the world. There are more reports from COVID-19 vaccines since 2021 than the total number of reports on all vaccines over the last 30 years. VAERS (US), v-safe (US), EUDRA (Europe), CDSCO (India), PMDA (Japan), and TGA (Australia) are all showing similar reports.
It is important to note that the US did not take the AstraZeneca vaccine because health officials publicly criticized AstraZeneca for using “outdated information” to show an incomplete view of the shot’s effectiveness. There were also concerns about the time between shots being extended. It has never been used in the US, especially after more than a dozen countries, mostly in Europe, temporarily suspended giving out the shot after reports linked it to a rare blood clotting disorder called vaccine-induced thrombotic thrombocytopaenia (VITT). (https://pubmed.ncbi.nlm.nih.gov/33987882/)
In 2021, AstraZeneca and its partners have released for supply two billion doses of their COVID-19 vaccine to more than 170 countries across every continent on the planet. More than 80% of those in India who took an injection were given the AstraZeneca vaccine.
How FOI Requests work
FOI provides an opportunity to develop a relationship with the public based on openness and transparency. Information Commissioner’s Office (ICO) states:
- Openness is fundamental to political health of a modern state.
- Public authorities spend money collected from taxpayers and make decisions that can significantly affect many people’s lives.
- Access to information helps the public make public authorities accountable for their actions and allows public debate to be better informed and more productive.
- Unnecessary secrecy in government leads to arrogance in governance and defective decision-making.
The principle behind the FOI Act is to release information unless there is a good reason not to.
Therefore, information can fall within an exemption, but public interest can favour disclosure.
We believe that the public interest test can be applied to this FOI, and that test is the safety of the nation’s health.
Six Members of the European Parliament (MEPs) held a press conference, on 11 October 2022, where MEP Francesca Donato (Italy), MEP Cristian Terhes (Romania), MEP Virginie Jeron (France), MEP Sylvia Limmer (Germany), MEP Ivan Sincic (Croatia), and MEP Christine Anderson (Germany) gave their reactions when Albert Bourla, CEO of Pfizer, failed to attend the meeting in person, but sent a representative in his place.
Dutch MEP Robert Roos asked the Pfizer representative a straightforward question about the company’s knowledge of prevention of infection and transmission prior to marketing and got a surprisingly straight ‘No.’ On 14 October 2022, European Union (EU) officials confirmed in a brief statement an “ongoing investigation into the acquisition of Covid-19 vaccines in the European Union.” They added that the case follows “extremely high public interest” around the issue of contracts, though declined to share any other details.
In Europe and around the world, many questions are being asked about the COVID-19 vaccines. and this will continue until answers are provided.
Conclusion
The UK government has invested millions of taxpayer’s monies to develop and market the AstraZeneca COVID-19 vaccine product. The government has coerced millions of people, a large percentage of its population, to be injected with the liability-free vaccine, and, therefore, citizens of the UK require complete government transparency, not suppression of information. It would show utter contempt for our democracy if the British people were denied access to the AstraZeneca data and information.
The medical and scientific community and the public have a substantial interest in reviewing the data and information underlying the MHRA’s approval of the AstraZeneca vaccine. Reviewing this information will settle the ongoing public debate regarding the MHRA’s review process. If their due diligence has been thorough, then releasing this data should confirm their oft-repeated declaration that the AstraZeneca vaccine is safe and effective, thus providing reassurance.
The AstraZeneca COVID-19 vaccine uses a brand-new, investigational technology that now has been injected into many millions in the UK, and a greater understanding of the data is required. The public’s need for this information is urgent given the fact that the COVID-19 vaccination programmes are continuing. We hope to receive a positive response to this request.
Update: Initial Response from MHRA
MHRA sent a response to PJH Law on 9 November 2022, and it states:
“This information request in its current format would be exempt under s12 or s14.
Section 12
Section 12 applies when the cost exceeds then limit of 24 hours to determine if the information is held, locate, retrieve, and extract the information. We estimate the time taken to conduct the above activities to be in excess of 36 hours.
Section 14
A Section 14 refusal, can be used in situations where handling multiple requests or a single request, would lead to a grossly excessive burden being placed on the public body or institution. We expect that this burden would be incurred due to the need to read, consider, and apply redactions, to the vast array of regulatory material encompassed by the request. We expect the time taken to conduct redactions to be >300 hours. We suggest that your client considers a refinement of their request before a formal refusal notice is issued.”
Though information can fall within an exemption, the public interest can favour disclosure. Ms. Grainger believes that the public interest test can be applied to this FOI and that test is the safety of the United Kingdom’s citizens’ health.
In the US, the FOIA request sent to the FDA to release all pre-licensure Pfizer data was challenged because of the amount time and number of people required to release the documentation. Yet, the judge ruled that release of the data was in the interest of the public and required the FDA to obtain the resources to make the release happen in a timely manner.
MHRA claiming that it will take too much time and effort is difficult to understand since they completed a “robust review” (their own words) in only three months of all the AstraZeneca data supplied from end September 2020.
Ms. Grainger is now exploring ways of obtaining the required information from MHRA.



