UK Children Overrepresented in Post-marketing Adverse Events while the FDA Downplays Myocarditis in 5-Year Pfizer Clinical Trial
The initial release of the Pfizer trials and post-marketing documentation allowed millions of concerned Americans to see what Pfizer did to ensure vaccine safety and efficacy. It gave people worldwide an opportunity to assess their data methods even as Pfizer fought hard to trickle these telling documents out over a half-century. Some findings coincided too with the FDA’s eager support of rolling out vaccines to children. This, even as their sources, the CDC, failed in data integrity while amping up SARS-COV-2 risk assessments tied to myocarditis in children.
For the Pfizer post-marketing literature, minors (<=17) represented 175 adverse reports. 34 of those reports were generated for children under 12 years of age.1In Table 3, Safety Concerns (pg. 9), included: “Use in pregnancy and lactation; Use in Pediatrics Individuals < 12 Years of Age; Vaccine Effectiveness.” From Table 6, Description of Missing Information (pg. 12), reported a laundry list of serious consequences for pregnant women and minor children.
The key highlights of this table for pregnant women and infants were2:
- “Number of cases: 413a(0.98% of the total PM dataset); 84 serious and 329 non-serious; • Pregnancy outcomes for the 270 pregnancies were reported as spontaneous abortion (23), outcome pending (5), premature birth with neonatal death, spontaneous abortion with intrauterine death (2 each), spontaneous abortion with neonatal death, and normal outcome (1 each). No outcome was provided for 238 pregnancies (note that 2 different outcomes were reported for each twin, and both were counted).
- 4 serious fetus/baby cases reported the PTs Exposure during pregnancy, Fetal growth restriction, Maternal exposure during pregnancy, Premature baby (2 each), and Death neonatal (1).
- 116 cases reported exposure to vaccine during breastfeeding (PT Exposure via breast milk) without the occurrence of any clinical adverse events.”
- 17 cases of the infant exposure to vaccine through breast milk resulted in a variety of clinical events, such as: rash (4), vomiting (2), diarrhea (2), and allergy to the vaccine (1).
Table 6 was more alarming for those minors administered the Pfizer mRNA vaccine. Highlights were:
- Number of cases: 34d(0.1% of the total PM dataset), indicative of administration in pediatric subjects <12 years of age;
- Country of incidence: UK (29), US (3), Germany and Andorra (1 each);
- Cases Seriousness: Serious (24), Non-Serious (10);
- Gender: Females (25), Males (7), Unknown (2);
- Age (n=34) ranged from 2 months to 9 years, mean = 3.7 years, median = 4.0;
- Case outcome: resolved/resolving (16), not resolved (13), and unknown (5).
- Of the 132 reported events, those reported more than once were as follows:
o Product administered to patient of inappropriate age (27, see Medication Error)
o Off label use (11) Pyrexia (6), Product use issue (5)
o Fatigue, Headache and Nausea (4 each)
o Vaccination site pain (3)
o Abdominal pain upper COVID-19, Facial paralysis, Lymphadenopathy, Malaise, Pruritus and Swelling (2 each).
Pregnancies impacted with spontaneous abortion equaled 8.5%, a significant loss for those mothers-to-be. In the footnote (d) on page 15, “Upon review, 28 additional cases were excluded from the analysis as the data reported (e.g. clinical details, height, weight, etc.) were not consistent with pediatric subjects.”3
In Table 7, AWSI Evaluation for BNT162b2, footnote (j) noted that, “UK MHRA described a 1-year-old subject who received the vaccine, and had left postauricular ear pain that progressed to left-sided Bell’s palsy 1 day following vaccination that had not resolved at the time of the report.”4[My emphasis.]
However, this did not trigger any significant misgivings for Pfizer. Their blunt analysis was: “The data do not reveal any novel safety concerns or risks requiring label changes and support a favorable benefit risk profile of to the BNT162b2 vaccine.”5 Then, Pfizer ended the 38-page report with 9 pages of “adverse events of special interest.”
A few of those listed, tied to infants, were:
- Ohtahara Syndrome6(Page 32: Early infantile epileptic encephalopathy with burst-suppression) • Infantile enterocolitis7(Page 31: Autoinflammation with infantile enterocolitis)
- Kostmann Syndrome8(Page 34: Infantile genetic agranulocytosis)
- West Syndrome9(Page 34: Infantile Spasms)
Meanwhile, the FDA in late 2021 reported Pfizer’s trial on kids had, “No cases of myocarditis/pericarditis, anaphylaxis, or Bell’s palsy/facial paralysis/facial paresis, or MIS-C10 were reported in the Study C4591007 5 to <12 years of age safety expansion group as of the data cutoff date (08 October 2021).”11,12 [My emphasis.] This study (until May 5, 202613) is funded by Pfizer.14
More tellingly though, the U.S. FDA downplayed myocarditis while also attributing it to a SARS-COV-2 infection. “The risk of myocarditis for children <16 years of age was recently reported to be >30 times higher for those infected with SARS-CoV-2 than for those who have not been infected. The risk of myocarditis is substantially lower after vaccination than it is after COVID-19 in populations where it has been evaluated, underscoring the benefits of vaccination despite the risk of this rare vaccine-associated adverse event.”15 [My emphasis.]
However, the Pfizer vaccine has been notable for its lack of ability to stop infection16, while introducing adverse events and long-term impacts no one can completely assess at this point. The Pfizer C4591007 clinical study reflects that with an end point in 2026. Moreover, recent data from third quarter of 2021, in the United States, showed substantial increases in mortality rates for the 25–44-year-old age range.17,18 Pfizer has approximately 60% of the U.S. market for COVID-19 vaccines, resulting in 1.4 billion doses as of August 31, 2021.19
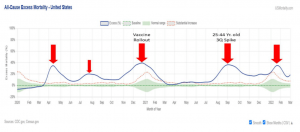
Insurance companies are aware of these numbers20,21 and are discovering the CDC failed as well to release relevant data22 and manipulated (overstated) child deaths. Recently, the CDC, removed 24% of pediatric deaths tied to COVID.23,24,25,26 The FDA paper above cited the CDC, using CDC data links in 19 of its 68 references.27 The FDA document (page 8-9), argued based on CDC data that was malapportioned and misrepresented for many
months. This while hardly convincing with the CDC data, saying that “the pediatric burden of COVID-19 likely exceeds that of seasonal influenza.”28 (My emphasis.) But ignoring other data signals far more consequential.
In all, the story regarding Pfizer, its study data, both internationally, and in the United States, should be under constant scrutiny with the release of each new batch of court-ordered documents.
References
1 https://phmpt.org/wp-content/uploads/2021/11/5.3.6-postmarketing-experience.pdf (page 7)
2 https://phmpt.org/wp-content/uploads/2021/11/5.3.6-postmarketing-experience.pdf (page 12)
3 https://phmpt.org/wp-content/uploads/2021/11/5.3.6-postmarketing-experience.pdf (page 15)
4 https://phmpt.org/wp-content/uploads/2021/11/5.3.6-postmarketing-experience.pdf (page 25)
5 https://phmpt.org/wp-content/uploads/2021/11/5.3.6-postmarketing-experience.pdf (page 28)
6 https://pubmed.ncbi.nlm.nih.gov/14734930
7 https://rarediseases.org/rare-diseases/autoinflammation-infantile-enterocolitis/
8 https://pubmed.ncbi.nlm.nih.gov/17537008/
9 https://www.epilepsy.com/learn/types-epilepsy-syndromes/infantile-spasms-west-syndrome
10 https://www.cdc.gov/mis/hcp/index.html (multisystem inflammatory syndrome in children)
11 https://www.fda.gov/media/153409/download (page 46)
BNT162B2 [COMIRNATY (COVID-19 VACCINE, MRNA)] VACCINES AND RELATED BIOLOGICAL PRODUCTS ADVISORY COMMITTEE BRIEFING DOCUMENT. 26 October 2021.
12 https://clinicaltrials.gov/ct2/show/NCT04816643 (Phase 1/2/3 Study)
13 https://clinicaltrials.gov/ct2/show/NCT04816643
14 https://www.nejm.org/doi/full/10.1056/nejmoa2116298 (Archive: https://archive.ph/mZhYY#selection-5493.0-5535.185)
15 https://www.fda.gov/media/153409/download (page 15)
16 https://www.nytimes.com/2022/02/28/health/pfizer-vaccine-kids.html
17 https://gettr.com/post/pztrv5cafc
18 https://www.usmortality.com/excess-percent
19 https://www.fda.gov/media/153409/download (page 8)
20 https://www.zerohedge.com/political/insurance-companies-note-jump-death-payouts-amid-40-rise-among-prime-age americans
21 https://archive.ph/eyCRS – “Insurance Companies Note 40 Percent Rise in Deaths” Epoch Times 22 https://www.nytimes.com/2022/02/20/health/covid-cdc-data.html
22: Boehmer TK, Kompaniyets L, Lavery AM, Hsu J, Ko JY, Yusuf H, et al. Association Between COVID-19 and Myocarditis Using Hospital Based Administrative Data – United States, March 2020-January 2021. MMWR Morb Mortal Wkly Rep. 2021;70(35):1228-32.
23: Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, Ohana R, et al. Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N Engl J Med. 2021.
23 https://www.yahoo.com/video/cdc-reports-fewer-covid-19-204027980.html (https://archive.ph/gWKzS#selection-795.0- 799.90 )
24 https://web.archive.org/web/20220317153346/https://covid.cdc.gov/covid-data-tracker/#demographics 25 https://www.washingtonexaminer.com/news/reported-pediatric-covid-19-deaths-plummet-24-after-cdc-fixes-coding logic-error
26 https://twitter.com/m_scribe/status/1503914736236257284
27 https://www.fda.gov/media/153409/download (pages 76-82)
28 https://www.fda.gov/media/153409/download (page 8)



