NAATs Used in Pfizer Clinical Trial to Test Asymptomatic Patients Despite Not Being Approved (or Even Emergency Use Authorized) for Asymptomatic Testing.
Nasal swab nucleic acid amplification tests (NAATs) are “a type of viral diagnostic test for SARS-CoV-2, the virus that causes COVID-19.” [https://www.cdc.gov/coronavirus/2019-ncov/lab/naats.html and https://www.fda.gov/consumers/consumer-updates/covid-19-test-basics]
Although certain NAAT tests have now been authorized for emergency use (EUA) for screening purposes (in asymptomatic persons), the three NAAT tests used at the time of the pivotal Pfizer trial did not have this as an “intended use.” Rather, the respective “Intended Use[s]” were only for “individuals suspected of COVID-19” or “individuals who meet COVID-19 clinical and/or epidemiological criteria.” [https://www.cdc.gov/coronavirus/2019-ncov/lab/naats.html, https://www.cepheid.com/Package Insert Files/Xpress-SARS-CoV-2/Xpert Xpress SARS-CoV-2 Assay ENGLISH Package Insert 302-3750 Rev. G.pdf, https://www.molecular.abbott/content/dam/add/molecular/products/infectious-disease/realtime-sars-cov-2-assay/Abbott RealTime SARS-CoV-2 Assay Package Insert.pdf, and https://web.archive.org/web/20200316175553/https://www.fda.gov/media/136049/download]
To receive vaccine in Pfizer’s clinical trial, individuals had to be asymptomatic. Subjects were checked for “current symptoms that could represent a potential COVID-19 illness.” The NAAT test was performed “at the time of each dose” to provide evidence of no current infection for the subsequent efficacy analysis. [https://docs.house.gov/meetings/IF/IF02/20200930/111063/HHRG-116-IF02-20200930-SD007.pdf]
In light of the then “Intended Use” of the respective package inserts, I ask two questions of the Food and Drug Administration (FDA) regarding the use of NAAT testing in asymptomatic subjects in the pivotal Pfizer COVID-19 vaccine trial. Please see my March 17, 2023, letter below to Dr. Peter Marks, Director of the Center for Biologics Evaluation and Research (CBER) within the Food and Drug Administration.
March 17, 2023
Dear Dr. Marks,
In organizing my files, I recently stumbled across some notes of mine following the first Emergency Use Authorization (EUA) of the COVID-19 vaccines in early December 2020. For some reason unclear to me, I did not share these concerns with you then, but I am now.
My questions:
1) Were the nasal swab nucleic acid amplification test (NAAT) package inserts’ “Intended Use,” i.e., “individuals suspected of COVID-19,” appropriately followed in the pivotal Pfizer COVID-19 vaccine trial?
2) If not appropriately followed, what is the impact on the EUA authorization of the COVID-19 vaccines (and subsequent Biologics License Application [BLA] approval)?
As of September 3, 2020, “only a few tests [had] received EUA for screening asymptomatic individuals.” 1 Most of the COVID-19 tests in the United States had only received EUA for testing of symptomatic individuals “(ie, for diagnostic testing).” 1 This was the case for the lab tests which had “not been FDA cleared or approved” to “detect SARS-CoV-2” in the Pfizer-BioNTech vaccine trial subjects. Today, the three NAAT tests remain under EUA. 2,3,4,5,6,7
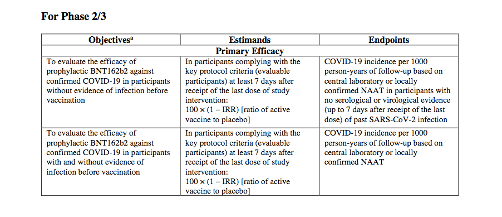
Yet both of the two primary efficacy endpoints in this pivotal trial rely on incidence of COVID-19 in participants “without evidence of [SARS-CoV-2] infection before vaccination” (subjects would naturally be asymptomatic with lab tests to ‘confirm’): 2,3,4
If symptomatic, the subject’s vaccination was to be delayed until “the [following] condition(s) has/have resolved”: “Current febrile illness (body temperature ≥100.4°F [≥38°C]) or other acute illness within 48 hours before study intervention administration. This includes current symptoms that could represent a potential COVID-19 illness.” 2
The infection status prior to vaccination was determined as follows: “…to qualify for the first primary efficacy endpoint evaluation, individuals needed to have no evidence of prior or current infection before each dose. And that was determined either by obtaining a swab at the time of each dose, to identify evidence of SARS-CoV-2 by nucleic acid amplification testing [NAAT], or obtaining a blood specimen for N-antigen antibodies at the time of the first dose to indicate evidence of prior infection that may have preceded vaccination by months.” 3,5
In my opinion, by using EUA lab tests not “FDA cleared or approved” in an investigational vaccine trial, a ‘study within a trial’ is effectively created; such a study “must not affect the scientific integrity of the host trial, its rationale or outcome measures.” 6
But back to the main focus of this letter, here are the stated “Intended Use[s]” of the respective NAAT package inserts employed in the asymptomatic trial subjects prior to vaccination: 2,4
- Intended Use: Cepheid Xpert Xpress SARS-CoV-2: individuals suspected of COVID-19 by their healthcare provider 7
- Intended Use: The Abbott RealTime SARS-CoV-2 assay: individuals suspected of COVID-19 by their healthcare provider 8
- Intended Use (note: Intended Use revised May 2021, see screenshot below): cobas® SARS-CoV-2 : individuals who meet COVID-19 clinical and/or epidemiological criteria 9,10

I look forward to your response, Dr. Marks, and appreciate acknowledgment of receipt of this letter,
Sincerely,
Carol Taccetta, MD, FCAP
References
2 https://docs.house.gov/meetings/IF/IF02/20200930/111063/HHRG-116-IF02-20200930-SD007.pdf
3: https://www.youtube.com/watch?v=owveMJBTc2I
4 https://www.fda.gov/media/144245/download
5 https://fda.report/media/144859/VRBPAC-12.10.20-Meeting-Transcript.pdf
6 https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-018-2535-5
9 https://www.fda.gov/media/136049/download
10https://web.archive.org/web/20200316175553/https://www.fda.gov/media/136049/download
One of our country’s most important freedoms is that of free speech.
Agree with this essay? Disagree? Join the debate by writing to DailyClout HERE.



