Part 1: The Alarming LNP (Lipid Nanoparticles) History You Haven’t Been Shown – the LNP Developers’ Own Studies Dating Back 20 Years
Introduction and Executive Brief sent to lawmakers & state investigators this summer.
The below work was written from March to May 2023; after working with attorneys and physicians to bring this information to the attention of our lawmakers, the below introduction and eight-page Executive Brief/synopsis accompany a 90-page executive summary that was sent to lawmakers, and more through the summer. These writings include references, quotes, and citations (last page) to the manufacturer’s and LNP developers’ published writings, which show their own concerns with the LNPs published as late as July 2020, just months before the EUA approval of the Covid-19 mRNA LNP-based vaccines. It also provides studies from the LNP development (and LNP developers) and extensive studies and documentation in medical journals on the pre-pandemic known reactions to the lipids in drugs that Pfizer lists as having similar lipid nanoparticles in its own internal documents and more. This series will be presented in several sections like the Twitter Files; these are the LNP Files.
###
Synopsis and Executive Brief to “Executive Summary on Sars-CoV-2 mRNA Vaccine (injectable therapies) Lipid Nanoparticles (LNPs) and Known Pre-pandemic Lipid and Liposomal CARPA (Complement Activated Reaction PSEUDO ALLERGY) and mRNA Covid-19 Vaccine Adverse Events, Myocarditis, and Autoimmunity”
May 2023 – Heather Hudson
Introduction:
1. In 2020, the Lipid Nanoparticle was a little-known and unknown science according to the Pfizer Covid-19 mRNA vaccine LNP co-developer: When the Covid-19 mRNA vaccines were approved under the Emergency Use Authorization (EUA), the consumer could not have known about these highly specific areas of nanomedicine and nanotechnology. The LNPs, studies, and development had been a little-known or niche area of science and medicine that was struggling to find its footing, funding, and success in treatment (largely due to safety issues (as seen in peer-reviewed articles within the attached Executive Summary)). Nano lipids were mainly used as carrier lipids in cancer drugs. In some of the drugs, patients were pre-medicated to avoid the lipid adverse reactions. LNPs had little recognition, as seen here, in this Cell Magazine article in which Acuitas labs co-founder and Covid-19 mRNA-LNP vaccine LNP co-developer Peter Cullis is interviewed, in 2022, and states, “Then in November [2020] … lipid nanoparticles became really popular. Nobody had ever heard of a lipid nanoparticle before, but in the last year and a half, they certainly have.”[11] More information can be found on the illusive nature of the use of pre-pandemic nanomedicine in references [12] and [23], and more can be found in quoted material within the attached Executive Summary.
2. What is an LNP? The lipid nanoparticle (LNP) can be thought of like a bubble that surrounds the drug or therapy (payload) that is to be delivered in the cells (for example mRNA gene therapy). The LNP bubble shape forms as numerous cone shaped individual lipid molecules attract to one another to form a bubble shape with a hollow center, which then houses the payload which can also include more LNPs along with gene therapy such as mRNA, siRNA, etc.
3. What do LNPs do? The LNPs provide a means for the therapy or drug to be carried inside the LNP bubble to travel in our body and enter our cells that are naturally surrounded by their own lipid layer. The cells allow the LNP in via an exchange of charges of the components when the LNP lipid and the natural cell lipid join. Inside the cell, the LNP falls apart and releases its payload. As seen in the medical studies sometimes the LNPs do not enter the cell (as they were intended to), or they are not adsorbed (due to adsorption rates and possibly the biomolecular or protein corona as seen below) and they can circulate in the body instead of entering the cell. Also, per the medical studies, sometimes the LNPs can fall apart before being taken up by the cells and the lipids circulate in the body. For the LNPs that enter cells, according to this study, “The apparent terminal t½ in plasma likely represents the re-distribution of the respective lipids from the tissues into which they have distributed as the LNP back to plasma where they are eliminated.”[44][75]
4. What is PEG? The LNP capsules often contain PEG (Polyethylene Glycol) which is an emulsifier that helps to keep the lipids stable and is meant to prolong the life of the LNPs and to stabilize the LNP bubble structure. Some people have a true allergy to PEG.
5. PEG and LNP safety issues: PEG is found in the medical literature to be immunogenic, and aside from PEG, the shape, size and biophysical properties (biomolecular corona, etc.) of the LNPs can elicit unwanted immune response which can be seen through the liposomal and LNP development history as the body mistakes nano sized molecules of a certain size for virus molecules. [72]
6. Unpredictable LNP adverse events: Beyond PEG allergy, PEGylated LNP molecules have a separate history of adverse reactions that are unpredictable, such as Complement Activated Pseudo Allergies or CARPA, autoimmune activation, coagulation dysregulation and more which are detailed and referenced in the Executive Summary.
7. Pre-pandemic lipid adverse reactions – LNP and lipid adverse reactions are NOT just an allergy; they can be deadly and can elicit complement-activated immune dysregulation (autoimmune disorders, and more): Certain past liposomal and LNP drugs have required pre-treatment with steroids, antihistamines and more to prevent adverse events brought on by the complement system such as pseudo allergies, CARPA, pseudo-HSRs and IRs or infusion reactions. Some of these lipids are described as “similar lipids” to the Covid-19 mRNA vaccines LNPs by Pfizer. The patients who take the LNP carrier drug with “similar lipids” (to the Pfizer Covid-19 vaccines lipids) were pre-treated for these reactions in the clinical trials, and these patients for this drug are pre-treated upon administration of this drug to date. These specific reactions occur at a “very common” and “common” rate per the FDA fact sheet for this drug and the ADR, and they were known pre-pandemic to Pfizer/BioNTech mRNA and LNP co-developers. They were not warned of or listed by name in the FDA EUA patient and provider fact sheet.
8. Unrecognized high rate of reaction: In 2018, Janos Szebeni, who learned to make liposomes in a lab with (the now senior VP of BioNTech Katalin Kariko), conducts LNP animal studies and more. He co-authored the following article on the role of complement activation in HSRs [Hypersensitivity Reactions, AKA IRs, IRRS, Infusion reactions and CARPA (pseudo allergy)], which explains, “HSRs have been traditionally categorized in four groups, … However, it has increasingly been recognized that a substantial portion of acute allergic reactions, whose symptoms fit in Coombs and Gell’s Type I category, are actually not initiated or mediated by pre-existing IgE antibodies. These reactions are known to be “pseudoallergic” or “anaphylactoid.” There are estimates that pseudoallergy may represent as high as 77% of all immune-mediated immediate HSRs…, implying hundreds of thousands of reactions and numerous fatalities every year… Many of these reactions involve the activation of the complement system, an essential humoral arm of innate immunity. Complement activation-related pseudoallergy (CARPA) is linked to adverse events evoked by several liposomal and micellar formulations, nanoparticles, radiocontrast agents, and therapeutic antibodies…”[81]
9. Relatively unknown reactions outside of the nanomedicine field: In this 2020 Science magazine article, written just after the Covid-19 mRNA vaccines saw at least eight people develop severe allergy-like reactions, it states, “Szebeni says the mechanism behind PEG-conjugated anaphylaxis is relatively unknown because it does not involve immunoglobulin E (IgE), the antibody type that causes classical allergic reactions. (That’s why he prefers to call them “anaphylactoid” reactions.) Instead, PEG triggers two other classes of antibodies, immunoglobulin M (IgM) and immunoglobulin G (IgG), involved in a branch of the body’s innate immunity called the complement system…”[6]
10. Pre-pandemic reactions resemble current reactions: This 2022 study states, “… the present study focused on the HSRs to Comirnaty, as it consists of PEGylated LNPs that resemble PEGylated liposomes, and “lessons” learned from nanomedicine research suggest that such nanoparticles (NPs) injected into the blood can cause so-called “infusion reactions” whose symptoms are very similar, or the same as those reported for the HSRs to LNP-mRNA vaccines …”[28]
11. Unpredictability and the Need for Premedication: This 2018 article, “Roadmap and strategy for overcoming infusion reactions to nanomedicines” by Szebeni et al. states, “Despite decades of research suggesting that the incidence of IRs depends on both pharmacodynamics and pharmacogenetics, it is largely unknown why some patients develop these reactions while others do not. The lack of uniform terminology and classification of the reactions further complicates the issue. As such, acute IRs cause substantial stress among patients and their families as well as care providers and regulatory agencies… Therefore, IRs in patients are currently managed by systemic administration of immunosuppressive, anti-pyretic, and anti-inflammatory medications before the infusion, during administration, or both…”[9] Again, these dysregulated immune reactions and adverse events have now been seen in the Covid-19 mRNA-LNP vaccines, including acknowledgement by the Mayo clinic and other physicians in case studies and more. [2][53-57][91] Reference [57] includes thirty-one cases of new-onset autoimmune disorders after Covid-19 vaccines; twenty-nine of these cases are attributed to Pfizer, and one of these cases was reported as having a fatal outcome, per the study.
12. PEG and LNP antibodies: This 2022 study, states, “The first injection of PEGylated drugs induces anti-PEG antibodies, which then bind and form an immune complex with the second dose of the PEGylated compound to activate the complement system. This results in the opsonization of PEG with C3 fragments and enhanced uptake by Kupffer cells in the liver and can result in altered drug pharmacokinetics and biodistribution and reduced drug efficacy in subsequent doses.” [86]
13. Circulation time pharmacokinetics, kinetics, and biodistribution in relation to adverse events: The LNP circulation time within the body and the biodistribution of the LNPs can play a part in these adverse reactions. Biodistribution studies and animal studies are crucial to determining the safety of the LNPs, as is seen in peer-reviewed studies within the Executive Summary.
14. Pfizer/BioNTech & Acuitas Therapeutics scientists show in their 2015 study that mRNA-LNP intramuscular injection depth has the ability to alter biodistribution and determine whether injections disseminate to the liver or stay in the arm/muscle: In 2015, a BioNTech VP and a co-mRNA developer, along with four scientists from the Acuitas Therapies lab (the lab that created the Pfizer Covid-19 vaccine LNPs) discovered that biodistribution of the mRNA-LNP formulation could be altered via the route of delivery of the mRNA-LNP in the body, but what is especially relevant is the depth of the muscle could determine whether the formulation distributed mainly to the liver or remained in the muscle tissue, as seen here in the study where it states, “Here, we show that in vivo administration of mRNA-LNP complexes by various commonly used injection routes displays different expression patterns and kinetics. Depending on the site of injection, both local protein production and dissemination to the liver occurs. Interestingly, we observed that with intramuscular injection, the depth into the muscle also determined whether the majority of protein produced was in the muscle, for superficial injections, or in the liver, for deep muscular administration (unpublished observations). Importantly, very low doses (0.005 mg/kg) of mRNA-LNPs could be translated for several days following the tested delivery routes… “[58] This information on the distribution of mRNA and LNPs in the body was not provided by Pfizer in their FDA EUA information to patients and providers.
See diagram and credit/link to this page if re-posting.
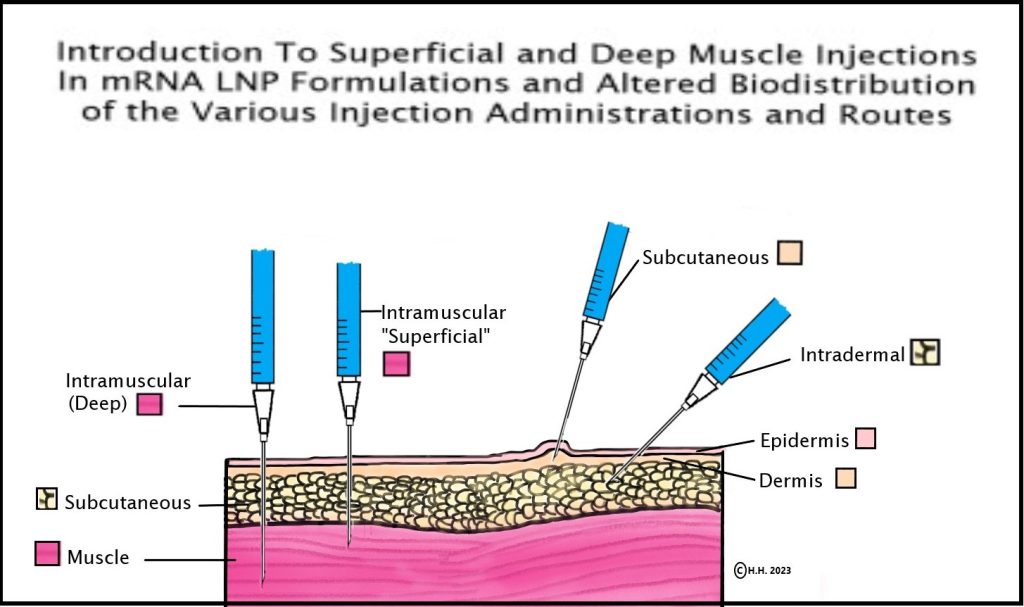
15. Similar Lipids 2015: In the same 2015 study above, the scientists compare the lipids used in the mRNA-LNP formulation to a similar drug which requires pre-treatment and assessment to circumvent known LNP complement activated adverse hypersensitivity like events, but the table of adverse events for this drug also includes “common” (rate of reaction) connective tissue disorders (AKA autoimmune adverse events). [58]
16. Similar Lipids 2022: In 2022, Pfizer also states these same lipids are similar to the Pfizer Covid-19 mRNA vaccines LNPs, also listing them as a “Relevant RNA therapy.”[2]
17. FDA and similar drug information: The 2007 FDA guidelines for drug labeling recommend that adverse drug reactions and adverse events in similar drugs should be included in drug labels “whether the adverse event is known to be caused by related drugs” and also “usage is associated with a clinically significant risk or hazard.”
18. 2020 FDA EUA application for Covid-19 vaccines, Nonclinical data section: The FDA states, “supportive data Available for the construct” are among the requirements to support first in human FIH clinical trials, toxicology data, and clinical data accrued with other products using the same platform may be possible to use to support FIH clinical trials”. Continued: “The purpose of nonclinical studies of a COVID-19 vaccine candidate is to define its immunogenicity and safety characteristics through in vitro and in vivo testing. Nonclinical studies in animal models… help identify potential vaccine-related safety risks and guide the selection of dose, dosing regimen, and route of administration to be used in clinical studies. The extent of nonclinical data required to support proceeding to first in human (FIH) clinical trials depends on the vaccine construct, the supportive data available for the construct, and data from closely related vaccines.”
19. Pfizer LNP co-developer on LNP safety and efficacy: In 2020, According to a study written three months before the FDA EUA Pfizer Covid-19 vaccine approval, the nature and biophysical features of the LNPs states, “even the same LNP tested in different animals, in different patients, or in the same individual over time might acquire different coronas, with potentially important implications for LNP clearance, target tissue accumulation, and efficacy” [72]. This study is co-written by the co-creator of the Pfizer LNP. The same 2020 study also states “In addition to the interference with cellular interactions, PEG can generate unwanted adverse effects such as complement activation, which may result in hypersensitivity reactions… and even anaphylaxis… PEGylation can trigger the production of antibodies that preclude the possibility of repetitive administrations… The use of PEG alternatives such as polysarcosines might be a possible solution to this problem…”[72]
20. The above 2020 study also states: “The physicochemical characteristics of LNPs change upon corona formation, which is specific to the biological environment in which particles are administered. As a consequence of this specificity, the same LNP will possess a different corona for each individual and each disease… This emphasizes, once more, that the “one size fits all” nanomedicine approach should be replaced with a more personalized framework…” [72] This study also shows that LNP adverse events have a factor beyond or separate from dose dependence in that the biophysical features and attributes of the LNP are associated with unwanted outcomes. Again, this study was co-authored by the Pfizer LNP co-developer three months before the Pfizer Covid-19 mRNA-LNP vaccine FDA EUA approval but this information was not shared to the consumer.
21. Other scientists in a similar 2020 LNP protein corona study: Another 2020 study on Biomolecular or Protein Corona efficacy and safety states, “In other words, identical NPs may form different protein corona profiles in patients with various diseases, significantly affecting the safety and therapeutic efficacy of the NPs across patients; however, those very differences in the protein corona that constitute a huge shortcoming for therapeutic applications can be exploited for disease detection ex vivo.”[79]
22. Pfizer’s internal 2022 documents state they do not understand the LNP immune responses: In their 2022 internal document Pfizer states, “…the immune responses to these lipids has not yet been fully understood.”

23. Unpredictability of the LNP shows Dose Dependence is an independent factor of in adverse events: It is well known, even by the American Cancer society and others that these reactions that are attributed to the LNPs can take place “immediately” “upon first contact”, or with a “delayed reaction”, (these reactions would then occur when very little LNP exists (or remains) in the body). Many of the reactions have taken place within the first few minutes of infusion and others have taken place weeks after administration.
24. A closer look at LNP “low doses”: In the “similar drug” clinical trials, it can be seen that 20.8 % of the trial participants had a reaction to the lower dose drug compared to 7.7% who had a reaction to the higher dose of the drug. [100]
25. Despite their studies and data, Pfizer scientists state the risk is negligible: In 2020, regarding anaphylaxis-like events (PEG and LNPs) seen within days of the EUA approval of the Pfizer mRNA-LNP vaccine, the BioNTech VP was quoted “…that given the low amount of lipid and the intramuscular administration, the risk was negligible.”[6].
26. A “safe dose” of LNPs in humans has not been established within a regulatory agency: In the Pfizer Covid-19 mRNA LNP formulations, the literature shows that these lipids are variable in several ways that affect safety, as seen in the 2020 “Biomolecular Corona” study seen in the Executive Summary and quoted below in item 27 and above in item 19 and 20. [72]
27. Pfizer and the LNP co-developers knew in 2015 and 2020 that the mRNA-LNP formulation could distribute outside of the muscle or injection site: The 2015 study introduced in items 14 and 15 above explain how the variation of the intramuscular injection could determine biodistribution into the liver, but consumers and their providers were not informed. Also, the 2020 study introduced in items 19 and 20 above explain how the Pfizer Covid-19 LNP co-developer knew the LNP had a variable outcome, that PEGylated lipids had known safety issues and that the “one size fits all” nanomedicine approach should be replaced with a more personalized framework” and it also states “However, once injected in a physiological environment, NPs interact with biological components and are surrounded by a protein corona (PC). This can trigger an immune response and affect NP toxicity and targeting capabilities.” [72]
28. The EMA detailed the Pfizer pre-clinical studies including the Wistar Han Rat Study No. 185350: On page 45 of the EMA Assessment Report for Comirnaty, it states, “PK studies with the two novel LNP-excipients ALC-0315 and ALC-0159”…“Following plasma clearance, the liver appears to be the major organ to which ALC-0315 and ALC-0159 distribute. The applicant has estimated the percentage of dose distributed to the liver to be ~60% for ALC-0315 and ~20% for ALC-0159. The observed liver distribution is consistent with the observations from the biodistribution study and the repeat-dose toxicology, both using IM administration.”[67 p. 46] [Note, PK= Pharmacokinetics.] [Note ALC-0315 and ALC-0159 are the two Pfizer non naturally occurring lipids.]
29. Pfizer knew about the possibility of autoimmune disease and the LNPs: In 2022, within Pfizer’s internal documents, we are also told that autoimmune activation was seen in a myocarditis case, and after explaining the known features of the LNPs that can bring about adverse events, this same internal paper states that “immune-mediated” events are the “most likely” cause of these myocarditis adverse reactions. [2] See also items 14 and 15 above where the adverse events known to the drug that Pfizer reports in their internal documents as having “similar lipids” (to the Covid-19 mRNA vaccines in 2022 and similar lipids to the 2015 mRNA-LNP studies co-authored by Pfizer scientists, mRNA and LNP co-developers) are attributed to connective tissue disorders. They reviewed and remarked on the safety studies of this similar drug in their own writings starting in 2015 and internal documents as late as 2022 but did not alert the consumer or their providers to the similarity of the lipids in this drug, which saw adverse events attributed to the lipids in the EMA studies and more.
30. In the 2022 Pfizer internal documents, the scientists express “well-known” vaccine-associated autoimmunity: This study states, “Vaccine-associated autoimmunity is a well-known phenomenon attributed most often to either the cross-reactivity between antigens or the effect of adjuvant. For example, HPV vaccine is linked to Guillain-Barre syndrome and other neuropathies (Watad et al, 2017). For COVID-19 mRNA vaccines, this matter becomes more complicated due to the nucleic acid formulation and presence of LNP as discussed above.” [2]
31. Pfizer also states that the animal models are “no good” in their internal document: Also, according to the medical literature, the animal models are not well suited: “there are no good animal models” to show potential myocarditis in humans. [2] This seemingly would include autoimmune myocarditis.
32. It should be considered that 45% of the 2021 pre-clinical trials for LNP-based drugs have safety warnings: The PDF from the FDA workshop in section FF considers “dispensing with” certain safety studies, yet this article points out the recent examples of the varying safety profiles of LNP drugs, found in this 2021 article, “Non-Immunotherapy Application of LNP-mRNA: Maximizing Efficacy and Safety,” a table of the current [2021] LNP-mRNA based protein replacement and rare disease pre-clinical studies show 20 LNP based drugs, pre-clinical studies underway. 45% of those (9) have safety warnings listed (as of that date); the others do not. Of the nine safety warnings, eight are for liver toxicity; three are for anti-drug antibodies, five show liver histopathology, and three list cytokines under the safety section. [63] Note: In the studies that did not have safety warnings in this table, it can be seen that some of these studies are not yet in the phase where safety warnings can be determined. In other words, it is possible the number of drugs mentioned in this study with a safety warning may amount to more than 45% of the drugs listed.
33. Reactions are little known to physicians: In 2018, a University of North Carolina study called “Physician Awareness of Immune Responses to Polyethylene Glycol‐Drug Conjugates” found that of the physicians polled, “Only 22% of physicians who prescribe PEGylated therapeutics are aware of APA. Only 35% of physicians who prescribe PEGylated drugs know that PEG is a part of that drug compound.” [note APA is Anti Peg Antibodies] The study continues on to say “These findings underline a need for improved awareness of APA. Physicians who prescribe PEGylated therapeutics should undergo targeted education. Given the low levels of awareness, it may be important to quantitate the efficacy of knowledge transfer from the research community to clinicians, especially on topics of patient safety. Finally, as the use of PEG in medicine becomes more prevalent, providers should closely monitor potential polypharmacy issues.” [93]
34. Potentially lesser known reactions: What is most notable about this study is the low number of physicians that were familiar with PEG antibodies, but most alarming is that in this study on awareness of PEGylated drugs adverse events, it did not mention CARPA (known to PEGylated drugs). This intelligent study was attributed in part to physicians in Microbiology & Immunology, Biomedical Engineering, Pharmacoengineering, and Molecular Pharmaceutics, as seen in the study. It provides numerous vital points on PEG ADAs (Anti-Drug Antibodies), highlighting the medical field’s lack of knowledge on the issues surrounding PEGylated drugs and their adverse reactions.
35. Reactions did not stop taking place in the Covid-19 mRNA-LNP Vaccines: Please consider that mRNA drugs and lipid-shell drugs and their gene therapy iterations struggled to meet safety standards and to gain approval for use for more than 20 years. Each endeavor took great work, and the industry made many attempts that failed throughout the gene therapy world, as seen in articles referenced within this paper. The latest attempt with FDA and EMA approval before the Covid-19 mRNA therapies/vaccines was the drug that Pfizer reports as having “similar lipids” (and lists as “Relevant RNA Therapy” in their internal documents), which required pretreatment during clinical trials, and it requires premedication upon administration to consumers to this day –due to the toxicity of the LNP– as seen in the medical literature and studies. This potential for LNP toxicity likely did not simply stop taking place in the Pfizer Covid-19 mRNA “similar lipid” vaccines.
For more information, please see my 90-page Executive Summary (posted as a series 1-10 over the next two weeks). Below is a sample of some of the information included in the next/coming sections:
- More data on Pfizer’s internal documents.
- Detailed and expanded information on the studies presented.
- Animal studies and a breakdown of the animal studies in comparison to Pfizer’s explanation of the animal studies in their internal documents recovered by Project Veritas.
- FDA and WHO guidelines for listing adverse events
- WHO guidelines for vaccine approval (as Pfizer utilized this data in their animal studies)
- Information on the need for accurate reporting of the adverse reactions and the rates of adverse reactions (which are not given to consumers or their providers in the Covid-19 vaccine FDA EUA).
- Detailed sections on the 2015 and 2020 studies by Pfizer mRNA and LNP scientists (which detail known distribution and safety issues that were also not given to the consumers or their providers).
- Detailed sections on Celebrex (in which Pfizer was “called out by the courts” for “overcoming the doubt of critics” by submitting only half of their safety study and the discussion on potential case relevance to COVID-19 vaccines.
- Links and reference material for the above data.
- And more.
Part 2 is available here.
Originally posted on A Mother’s Anthem Substack.
One of our country’s most important freedoms is that of free speech.
Agree with this essay? Disagree? Join the debate by writing to DailyClout HERE.



