“Infant RSV mRNA Injection Trials Paused Due to Safety Concerns”
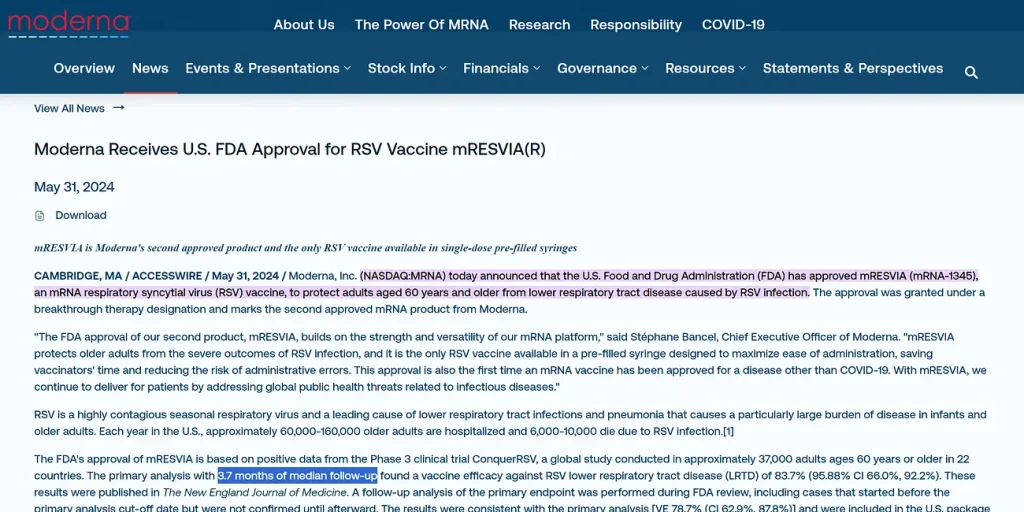
The FDA has just released a briefing document for the December 12, 2024, Vaccines and Related Biological Products Advisory Committee (VRBPAC) Meeting titled, Considerations for Respiratory Syncytial Virus (RSV) Vaccine Safety in Pediatric Populations. The document revealed that, in July 2024, a Phase 1 trial assessing the safety, tolerability, and immunogenicity of two Moderna RSV vaccine candidates (mRNA-1365 and mRNA-1345) in infants aged 5 to 8 months was paused following reports of five severe to very severe cases of lower respiratory tract infection (LRTI) caused by RSV:
During the study, an imbalance in severe RSV cases was identified, based on a pre-specified study stopping criterion, among participants 5 months through <8 months of age who received the lower mRNA vaccine dose. In Cohorts 3 and 4, five (5) cases (12.5% of participants) of clinically significant (CS) severe/very severe RSV were identified in the vaccine groups (all of whom had received 1 or 2 doses of a 3-dose schedule), compared with one (1) case (5% of participants) in the placebo group. The percentage of participants with symptomatic RSV disease in Cohorts 3 and 4 who progressed to severe illness was 26.3% in the vaccine groups compared with 8.3% in the placebo group.
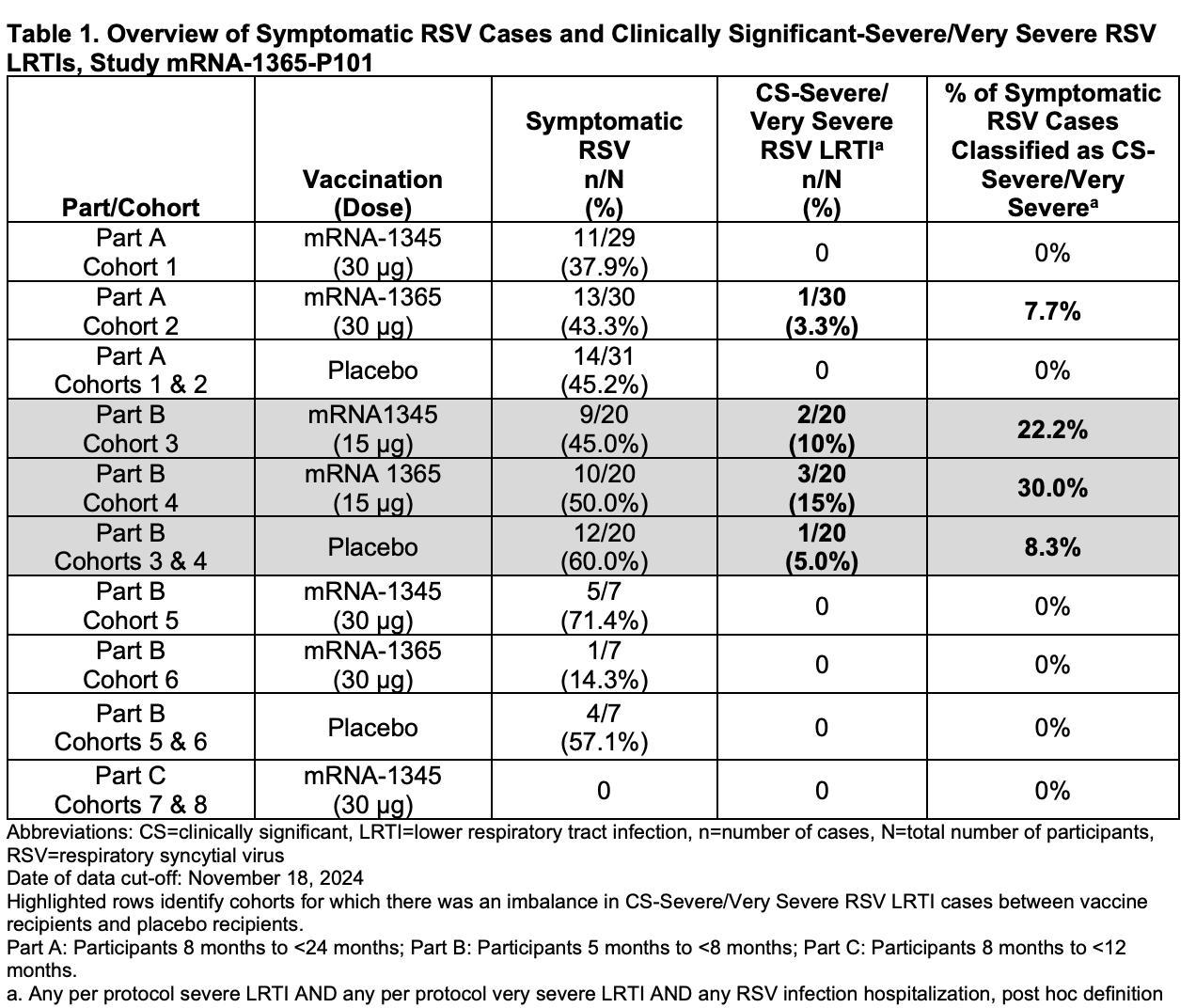
Of the six total severe cases (including one in the placebo group), five infants required hospitalization, and one required mechanical ventilation. The specific case details are presented in the following table:
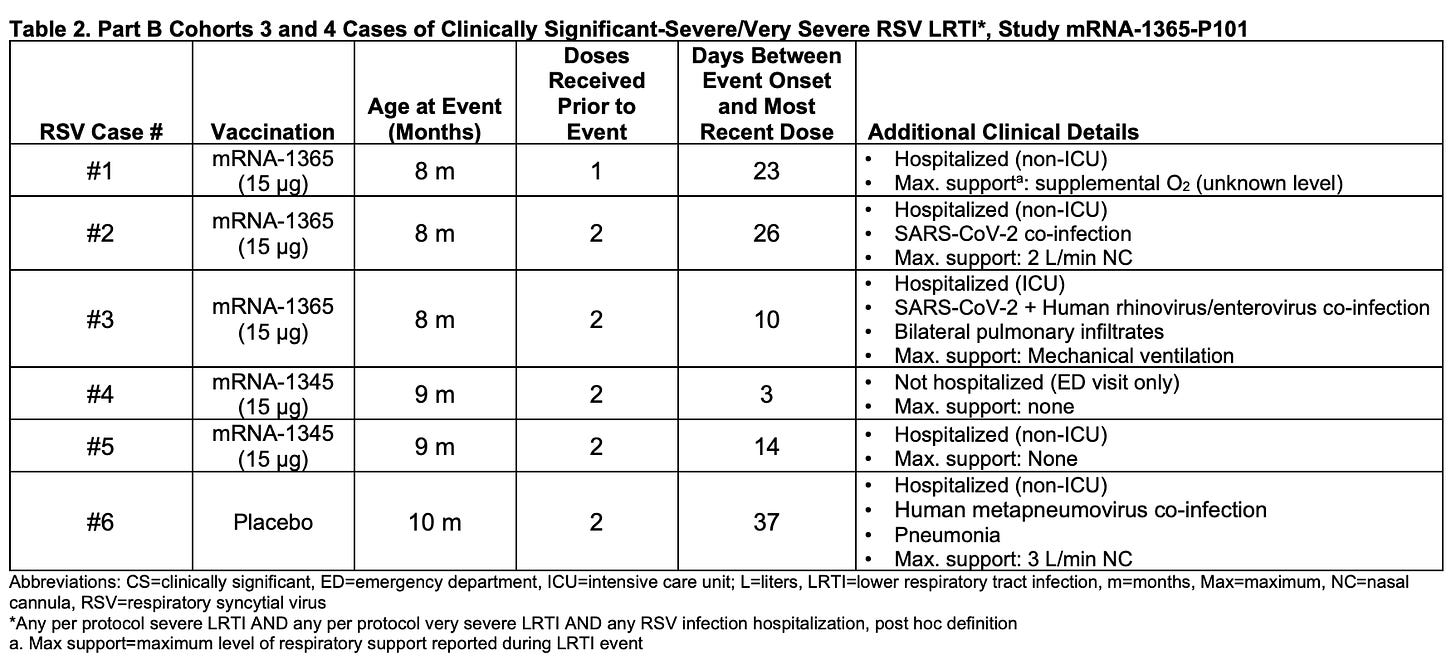
One of the candidates in the paused trial was mRNA-1345 (mRESVIA), which had already been approved by the FDA for adults aged 60 and older, despite the absence of genotoxicity, oncogenicity, or long-term safety studies:
This is yet another instance of mRNA injections failing to meet essential safety requirements. Rather than protecting infants from RSV, these novel injections seem to have worsened the severity of infections. Instead of persisting with the development and rollout of this flawed mRNA platform, our public health agencies should prioritize interventions that do not commonly result in serious adverse events.
_____
Follow DailyClout on Rumble! https://rumble.com/user/DailyClout
Please Support Our Sponsors
Birch Gold Group: “A Gold IRA from Birch Gold Group is the ultimate inflation hedge for your savings in uncertain times. Visit https://birchgold.com/dailyclout to see how to protect your IRA or 401(k).”
The Wellness Company: https://dailyclouthealth.com
Use code DAILYCLOUT for 10% off!
NativePath: “Electrolyte and sports drinks are doing more harm than good to your body. Stay healthy and hydrated with NativeHydrate… Visit https://nativehydrate.com/DailyClout to learn more.”
Patriot Mobile: “Visit https://patriotmobile.com/dailyclout for a FREE month of service when you switch!”
Order ‘The Pfizer Papers’ and Support Our Historic Work: https://www.amazon.com/dp/1648210376?&tag=skyhorsepub-20
Discover LegiSector! Stay up-to-date on issues you care about with LegiSector’s state-of-the-art summarizing capabilities and customizable portals. No researchers needed, no lobbyists, no spin. Legislation at your fingertips! Learn more at https://www.legisector.com/
One of our country’s most important freedoms is that of free speech.
Agree with this essay? Disagree? Join the debate by writing to DailyClout HERE.




