FOIA’d CDC Emails Reveal Disturbing Myocarditis Timeline Warranting Investigation: Different Messaging Internally Vs. Publicly About COVID-19 Vaccines and Myocarditis.

“We don’t have any evidence to suggest a signal or a safety problem for myocarditis[.]”
Dr. Tom Shimabukuro, CDC, April 19, 2021
Investigative work by two intrepid scientists, combined with Freedom of Information Act (FOIA) records, reveal a disturbing chronology during which the Centers for Disease Control and Prevention (CDC) “delayed reporting the incidence of myocarditis to the general public for three months after the first statistically significant signal appeared in the [Vaccine Adverse Event Reporting System (VAERS)] database…especially in young male Americans from 18 to 21 years of age.” (Jablonowski, K., & Hooker, B. S. (2022). Delayed Vigilance: A Comment on Myocarditis in Association with the COVID-19 Injections. International Journal of Vaccine Theory, Practice, and Research, 2(2), 651.1–651.4, https://doi.org/10.56098/ijvtpr.v2i2.61; https://tinyurl.com/2p9f523n) The delayed public notification also followed early 2021 warnings sent to the CDC from Israel and Australia about myocarditis in young people following mRNA COVID-19 vaccination.
Meanwhile, 112,000,000 unwitting Americans were exposed to a myocarditis risk they might not have chosen if fully informed. (Id.)
In this first of two articles, we relate in almost documentary format with little commentary the chronology from late February through May 2021 revealed by CDC emails to date, integrating three myocarditis safety signal data points identified by Jablonowski and Hooker. Though extant CDC emails about myocarditis predate February through May 2021, we focus on that period because it corresponds with when VAERS data, as identified by Jablonowski and Hooker, showed a myocarditis signal and with the date of CDC’s belated public announcement regarding post-COVID-19 vaccination myocarditis.
We claim the scientists’ work product, combined with content from the CDC emails, raise enough questions to justify investigating CDC personnel and possibly others for incompetence, negligence, and wrongdoing. We do not rush to judgment, because we recognize that facts which raise questions often have innocent explanations, investigations are intended to investigate, and they can serve to dispel suspicion as effectively as to confirm it.
Our second article will explore the prospects for state-level investigation and prosecution, if supported, including discussing which government entities could investigate and in what forums, who could be investigated and for what possible offenses (including reckless endangerment and official misconduct), federal supremacy clause immunity, jurisdiction and venue, and statute of limitations and tolling. We take this serial approach because we believe the CDC emails’ content should be publicly available without delay in a format more easily accessible than voluminous FOIA records.
Unless otherwise indicated, all dates below are from 2021, sometimes left in the text, either for emphasis or because 2021 is within a quotation.
CDC self-describes as “the nation’s leading science-based, data-driven, service organization that protects the public’s health[,]” (About CDC | About | CDC; https://tinyurl.com/39k5s5zm) which uses “the Vaccine Adverse Event Reporting System (VAERS) [as] a national early warning system to detect possible safety problems in U.S.-licensed vaccines[.]” (VAERS – About Us (hhs.gov); https://tinyurl.com/ythdxxth).
February 19.
VAERS reports “implicat[ed] myocarditis [as] causally connected to the COVID-19 vaccine in young males with greater than 95% confidence – the typical threshold beyond reasonable doubt.” (Jablonowski KD, Hooker B, et al., (2023) Lock the Doors: The Myocarditis Disaster and a call for the broad examination of the CDC and FDA [Food and Drug Administration], Medical Research Archives [online] 11(6), European Society of Medicine, 06/30/23 https://tinyurl.com/v7vf684m) (hereinafter “Jablonowski and Hooker”).
We turn to the emails, inviting readers to consider what the emails show CDC personnel discussed in advance of two dates, April 19, the date when post-COVID-19 vaccination pericarditis and likely myocarditis were addressed in a White House deputies meeting, and May 27, the date when the CDC publicly acknowledged risk of myocarditis related to the COVID-19 vaccines. (Reader note: we stand by our use of “appears,” “likely,” “may have,” and “suggests” because we are making reasonable surmisals from the emails’ content and context in FOIA records heavily redacted in places. We are open to challenge on our surmisals.)
February 28.
Personnel at the Israel Ministry of Health (IMOH) contacted CDC about “a large number of reports of myocarditis, particularly in young people, following the administration of the Pfizer vaccine.” (23-00056-for-2-24-2023-Close.pdf (childrenshealthdefense.org), p. 712) (for FOIA records cited readers will need to scroll to cited page numbers). CDC personnel discussed coordinating a response. (pp. 297-298, 666-667, 710-712).
March 3.
IMOH reached out to CDC again: “We are seeing a large number of myocarditis and pericarditis cases in young individuals soon after Pfizer COVID-19 vaccine. We would like to discuss the issue with the relevant expert at CDC.” (p. 668).

March 4.
Elaine R. Miller, RN, MPH, CDC Immunization Safety Office, asked: “Is anyone able to talk to this MD from the Israel Ministry of Health?” (pp. 666-667). Tom Shimabukuro, MD, MPH, MBA, CDC’s “vaccine safety team lead on the CDC COVID-19 Vaccine Task Force” (23-00056-for-2-24-2023-Close.pdf (childrenshealthdefense.org), 726), responded, though the content of his response is redacted. (p. 666).
Dr. Shimabukuro began “working with FDA to respond.” (p. 754) When contacted by a member of CDC’s VTF Global [CDC’s COVID19 Vaccine Task Force—Global Team], Miller stated “[t]here [was] no action required for VTF Global.” (pp. 753-754). A member of VTF Global differed: “It may be important for VTF Global to also be looped into these discussions given our global work. I am adding Laura Conklin to this thread as our POC for vaccine safety on the VTF Global Team.” (p. 754).
Soon, CDC and IMOH exchanged emails, a Zoom meeting appears to have been scheduled (pp. 668, 710-714, 721-726), during which it appears IMOH shared its myocarditis and pericarditis data with CDC. (p. 725) (“We will be happy to share our data.”). We emphasize, the emails strongly suggest IMOH shared its concerning myocarditis data with CDC in March 2021. At this writing, DailyClout does not have that IMOH data.

Within the CDC-IMOH emails, there is an undated Word memorandum titled “Summary of VAERS Report of myocarditis, pericarditis and myopericarditis following vaccination with mRNA COVID-19 vaccines” without an identified author. It states, in part: “This memo responds to questions posed from [IMOH] to the FDA and CDC. They are investigating a safety signal of myocarditis[]/myopericarditis in a younger population (16[]-30 years old) following administration of Pfizer-BioNTech Covid-19 vaccine.” (p. 714) (italics and bold added) “[IMOH] reports” “around 40 cases of this adverse event.” “A search of [VAERS]” “on February 23, 2021[,] revealed 27 cases (6 cases of myocarditis, 7 cases of myopericarditis, 14 cases of pericarditis).” (p. 714). The memo discusses search criteria used, review and categorization methodology, and inclusion and exclusion methodology, further stating: “[T]he reporting rate of myopericarditis following administration of the mRNA COVID-19 vaccines was low and estimated to be 0.7 per million doses of vaccine administered. However, the limitations of passive surveillance such as under-reporting, lack of a control group, missing and incomplete data make it challenging to assess causation. Thus, FDA has not made a final determination regarding the causality between myopericarditis and the mRNA COVID-19 vaccines. We will continue to monitor this outcome in active and passive surveillance.” (pp. 714-715).
March 8.
Western Australia state health personnel emailed CDC about myocarditis and other post-COVID-19-vaccination adverse events. (p. 700). As part of multi-recipient email discussions spanning through April 1, Dr. Shimabukuro provided the Australians with a draft manuscript of an article under review at Vaccine; information in an email, which takes up two thirds of a page when printed, to be kept “close hold[,]” all of which is redacted; and attachments comprised of “some ACIP COVID-19 vaccine safety technical subgroup presentations” to be “treat[ed]” “as confidential[.]” (pp. 698-703).

April 1.
John R. Su, MD, PhD, MPH, Vaccine Safety Team CDC COVID-19 Vaccine Task Force, sent an email with “RE: VAERS Review of Myocarditis/Myopericarditis for DoD [Department of Defense] Discussion Tomorrow” in the subject line, with an attachment titled “VAERS myopericarditis Vaccine 2021.pdf,” one recipient of which was Dr. Shimabukuro. (p. 526).
Oddly, about three months into the largest vaccine rollout and vaccination campaign in history, Theresa Harrington, MD, MPH&TM, Immunization Safety Office, CDC, sent a high-importance email (with a page and quarter of now-redacted text) with this subject line: “Can you please send VAERS definition of Serious [sic] to [David MCCORMICK | Medical Epidemiologist | Centers for Disease Control and Prevention, GA | CDC | Division of Vector-Borne Diseases | Research profile (researchgate.net)]?” (23-00056-for-2-24-2023-Close.pdf (childrenshealthdefense.org), pp. 894-896).
April 2 (week of).
“VAERS [had received] enough serious adverse event reports implicating a causal connection between the COVID-19 vaccine and myocarditis in young males with a 99.9% confidence.” (Jablonowski and Hooker) (hyperlink added).
April 6.
Dr. Grace Lee (Grace Lee – Stanford Medicine Children’s Health (stanfordchildrens.org) emailed CDC recipients, including Lauri E. Markowitz, MD (confex.com), Dr. Shimabukuro, and others, with “Signal eval considerations-[]feel free to comment/edit/share” in the subject line, stating “Hi Team, I started drafting just what came from the closed meeting but added some thoughts below.” (p. 962). The remaining over two pages of content is redacted.
Dr. Shimabukuro sent the agenda items for the “USG vax safety coordination call[.]” The five agenda items included these three: “DoD myocarditis case series preview[,]” “VSD [Vaccine Safety Datalink] and VA [Veterans’ Affairs] efforts for signal refinement for PE [pulmonary embolism] and AMI [acute myocardial infarction][,]” and “CDC update on CVST [cerebral venous sinus thrombosis] epi and CVST reports to VAERS[.]” (23-00056-for-2-24-2023-Close.pdf (childrenshealthdefense.org), p. 981).
April 11.
Dr. Jay Montgomery (likely a medical director by that name at the Defense Health Agency’s Immunization Healthcare Division’s North Atlantic Region Vaccine Safety Hub) (Dr. Jay Montgomery Details Importance of the Immunization Healthcare Division | Health.mil), sent Dr. Markowitz, and Julianne Gee, MD, a “slide deck for Monday’s VaST presentation on Myocarditis following COVID-19 Vaccination.” (23-00056-for-2-24-2023-Close.pdf (childrenshealthdefense.org), p. 695).
April 12.
Dr. Markowitz likely forwarded that slide deck in PDF format attached to an email she sent Dr. Shimabukuro, Dr. Su, and Karen Broder. (“I will send pdfs of presentations to the VaST [Vaccine Safety Technical] members[.]”) (pp. 693-695) (sent email to Dr. Shimabukuro, et al.) (p. 693).
April 13.
Dr. Markowitz sent an email informing Melinda Wharton, Dr. Lee, and Bob Hopkins that “DoD has submitted manuscripts of post-COVID-19 vaccine-associated myocarditis to both JACC [Journals of the American College of Cardiology] and JAMA Cardiology.” (p. 985).
April 15.
Dr. Shimabukuro received an email from Dr. Cody Meissner, Cody Meissner | Tufts Lyme Disease Initiative: “Writing to be sure you’re aware [material referred to in email redacted] and to ask if myocarditis has been reported in other vaccinees?” (23-00056-for-2-24-2023-Close.pdf (childrenshealthdefense.org), 916) Dr. Shimabukuro replied on April 17: “There have been reports of myocarditis following mRNA vaccines.” (p. 915) (emphasis added).

April 19.
Dana Meaney-Delman, MD, MPH, FACOG, then Chief, Infant Outcomes Monitoring Research and Prevention Branch, CDC, sent an email at 9:42 AM with “Pericarditis and Mrna [sic] vaccines” in the subject line, stating: “Ann S is on the deputies call with WH [White House] right now and is asking if there has been any signal with pericarditis and mRNA vaccines.” (CDC FOIA 23-00756 CDC responsive records.pdf – Google Drive, 302) She sent it to Dr. Shimabukuro and others. “Ann S” is likely Ann Schuchat, MD, former Deputy Director of CDC and a current GAVI Board Member.
Dr. Shimabukuro responded five minutes later: “DoD and the Israel MOH think they have a signal for myocarditis with mRNA vaccines, but there is potentially a lot of ascertainment bias in the DoD data. We don’t have any evidence to suggest a signal or a safety problem for myocarditis or pericarditis with mRNA vaccines from VAERS and VSD surveillance and FDA and VA have not detected any signals in their monitoring.” (p. 301) (italics added).

His “[w]e don’t have any evidence to suggest a signal or a safety problem for myocarditis or pericarditis with mRNA vaccines” is hard to explain, given the VAERS data by the week of April 2, “implicating a causal connection between the COVID-19 vaccine and myocarditis in young males with a 99.9% confidence.” (Jablonowski and Hooker). It is even harder to explain because Dr. Shimabukuro was included in at least some of the emails between CDC and both Australian and Israeli health personnel, shared information with the Australians, may have been on a Zoom meeting during which IMOH shared its myocarditis data with CDC (or privy to the data IMOH shared), may have read or even written the undated CDC internal memorandum discussing IMOH’s safety signal (“[t]hey are investigating a safety signal of myocarditis[]/myopericarditis”) (23-00056-for-2-24-2023-Close.pdf (childrenshealthdefense.org), p. 714), as well as being included in the DoD-myocarditis-related emails above. It is still harder to explain, given a later, May 14, email where Dr. Shimabukuro states “[w]e have been monitoring myocarditis/pericarditis as a VAERS adverse event of special interest from the beginning.” (CDC FOIA 23-00756 CDC responsive records.pdf – Google Drive, 275) (italics and hyperlink added). As an aside, readers can decide from the context of the exchange above whether it appears this was the first time some CDC personnel on the chain heard of the claimed association between COVID-19 vaccination and myocarditis/pericarditis.
Without knowing the content of the attachment titled “VAERS myopericarditis Vaccine 2021.pdf” Dr. Shimabukuro was sent on April 1 (23-00056-for-2-24-2023-Close.pdf (childrenshealthdefense.org), 525-526), it is indeterminate whether his “[w]e don’t have any evidence to suggest a signal or a safety problem for myocarditis or pericarditis with mRNA vaccines” statement is consistent therewith.
Perhaps there will be a later semantic disagreement about what constitutes a “safety signal,” despite the fact that it is clearly defined by Health and Human Services (HHS) in its “Guidance for Industry Good Pharmacovigilance Practices and Pharmacoepidemiologic Assessment,” but stating “[w]e don’t have any evidence to suggest” “a safety problem for myocarditis or pericarditis with mRNA vaccines” is not supportable, at best. We note Pfizer refers to myocarditis as a “signal” in its Summary Monthly Safety Report 5 for Active Substance: PF07302048 (BNT162b2) for the April 1 through 29, 2021, reporting period, (19736_S0335-M1_smsr-01apr2021-29apr2021.pdf (pdata0916.s3.us-east-2.amazonaws.com)), which it “evaluated and determined not to be [a] risk[.]” (p. 3). While it is unclear when CDC had access to that report, CDC had access to VAERS; and Pfizer’s determinations are open to challenge.
The five-minute lapse between the April 19, 9:42 AM email inquiry and Dr. Shimabukuro’s 9:47 AM response, and comparing the skeletal information in the inquiry, and the additional information in the response, suggests a phone call occurred in between, especially considering how fast his email was forwarded up the CDC chain of command after 9:47 AM. (23-00056-for-2-24-2023-Close.pdf (childrenshealthdefense.org), p. 301). Dr. Shimabukuro sent his assuaging email to intermediate recipients, one of whom forwarded it to two higher intermediate recipients, one of whom, Dr. Schuchat, forwarded it to CDC Director Dr. Rochelle Walensky, copying a second person, all of which happened in two minutes, 34 seconds. (p. 301).
Forty-one minutes later, Dr. Shimabukuro emailed: “We reviewed the VSD RCA [Rapid Cycle Analysis] data this morning and discussed the DoD data. We have substantial doses administered in younger age groups in VSD and don’t have a hint of a signal; it’s actually protective in the vaccinated concurrent comparator. We aren’t really sure why DoD thinks they have a signal[,]” (23-00056-for-2-24-2023-Close.pdf (childrenshealthdefense.org), pp. 308-309) (italics added), even though, as previously stated, by the week of April 2 “VAERS [had received] enough serious adverse event reports implicating a causal connection between the COVID-19 vaccine and myocarditis in young males with a 99.9% confidence[,]” (Jablonowski and Hooker) and notwithstanding the DoD dialog about myocarditis above.

That email also was sent up the CDC chain of command—less rapidly—with the final intermediary stating, “More reassuring[,]” in her forwarding email to Dr. Walensky, evoking the latter’s relieved response: “Much [reassuring]—great—thank you!” (CDC FOIA 23-00756 CDC responsive records.pdf – Google Drive, 308).
April 20.
Idaho Department of Health and Welfare personnel emailed CDC personnel Elaine Miller asking if CDC had received two Idaho myocarditis adverse event reports and whether CDC was “seeing any VAERS signals around second dose Pfizer and myocarditis? [sic] This is our third.” (23-00056-for-2-24-2023-Close.pdf (childrenshealthdefense.org), p. 798). Miller responded: “I did not find either of these reports in VAERS yet, but due to processing delays, VAERS may have received them but not processed them yet.” And “[t]o date, CDC vaccine safety monitoring systems have not found evidence of a concerning pattern of reports of myocarditis after COVID-19 vaccination.” (p. 797).
April 23.
“VAERS [had] received enough serious adverse event reports implicating a causal connection between the COVID-19 vaccine and myocarditis in young males with greater than 99.99% confidence – the bar for highest scientific scrutiny.” (Jablonowski and Hooker).
April 26.
Dr. Shimabukuro appears to initiate circulating a draft regarding myocarditis to Miller and others (“RE: Myocarditis” is the subject line). His email content and that of others in this chain are heavily redacted. (p. 851).
Dr. Shimabukuro sent an email to Dr. Thomas Clark, Division of Viral Diseases Leadership Bios | CDC, and other with “RE: Please read: Myocarditis” the subject line. All content, about a full page, is redacted. (23-00056-for-2-24-2023-Close.pdf (childrenshealthdefense.org), p. 945). That email appears to have been forwarded up the organizational chart, eventually to Dr. Walensky. This date saw a flurry of emails (including high importance emails) with “Myocarditis” in the subject line, the contents of which are all or partially redacted, apparently discussing a draft statement (pp. 848-854), likely for Dr. Walensky’s use at the press briefing the next day.
April 27.
In that press briefing, Dr. Walensky stated: “We have not seen a signal and we’ve actually looked intentionally for the signal in the over 200 million doses we’ve given.” (U.S. CDC has not seen link between heart inflammation and COVID-19 vaccines | Reuters; https://tinyurl.com/9va2tfvp) (italics added).
April 28.
Dr. Walensky emailed Abbigail Tumpey, then CDC’s Interim Director of Communications (Tumpey Named Vice President for Institute Communications (gatech.edu) with “RE: Myocarditis TPs [Talking Points]” in the subject line: “We need this for the presser – [where] are we in running this down?” (CDC FOIA 23-00756 CDC responsive records.pdf – Google Drive), p. 307). What was needed is now redacted. Ms. Tumpey’s response: “Below is a response and at the bottom are internal notes for the discussions with DoD.” Redactions follow, then “Notes from DoD discussion (not for public release, but for your awareness)[,]” (p. 306) with about a page and half of redactions. This is the first of at least three acknowledgments that there were two sets of information about COVID-19 vaccines and myocarditis: what was discussed internally at CDC, and what could be shared publicly.
Henry Walke, MD, MPH, emailed Dr. Walensky, copying Dr. Schuchat: “We will pull together talking point on myocarditis to share.” (p. 984). (23-00056-for-2-24-2023-Close.pdf (childrenshealthdefense.org))
An email exchange occurred between Dr. Su and Dr. Shimabukuro with “myocarditis cases” in the subject line, all content redacted, except for one statement by Dr. Shimabukuro: “We need to expedite these reviews and get ahead of the situation before it starts dictating our actions.” (italics added) (pp. 973-974).
Dr. Walensky was (still?) relying vicariously on the safety team. She emailed Dr. Meaney-Delman: “On the deputies call there was [about five lines redacted].” (CDC FOIA 23-00756 CDC responsive records.pdf – Google Drive, p. 314). Dr. Meaney-Delman: “Let me ask the safety team. You saw the summary from yesterday, right?” (p. 314).
April 28.
Talking points on myocarditis—including what is happening at DoD and in Israel—are prepared for Dr. Walensky. (p. 317).
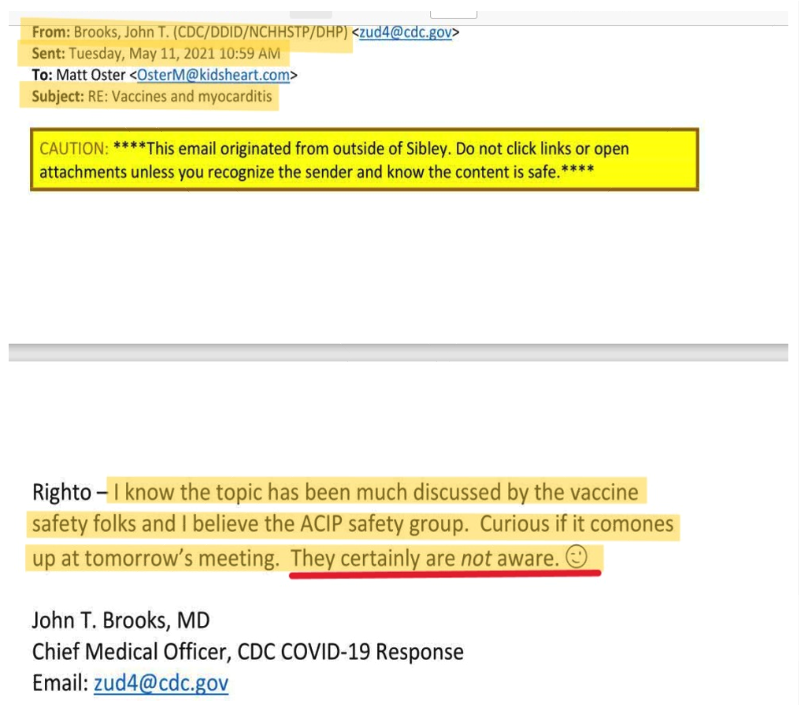
May 11.
Significantly the day before May 12, discussed below, and strangely, John Brooks, Chief Medical Officer, CDC COVID-19 Response, sent an email with “Vaccines and myocarditis” in the subject line, to Matt Oster, MD, (Atlanta Pediatric Research | Matthew Oster, MD, MPH | Faculty | People | Emory + Children’s + GT | Atlanta Pediatric Research Alliance (pedsresearch.org), stating: “I know the topic [of myocarditis] has been much discussed by the vaccine safety folks and I believe the ACIP [CDC’s Advisory Committee on Immunization Practices] study group. Curious if it [comes] up at tomorrow’s meeting. They certainly are not aware. [emoji omitted]” (CDC FOIA 23-00756 CDC responsive records.pdf – Google Drive, pp. 110-111) (italics in original). The strangeness is Dr. Brooks italicizing “not aware” and adding a knowing wink emoji at the end of his sentence. (pp. 110-111).
May 12.
Despite the then-available VAERS data, and notwithstanding all the myocarditis-related communication above, CDC recommended use of Pfizer’s COVID-19 vaccine in adolescents ages 12 and older. CDC Director Statement on Pfizer’s Use of COVID-19 Vaccine in Adolescents Age 12 and Older | CDC Online Newsroom | CDC; https://tinyurl.com/4fyxrhm2 (“CDC now recommends that this vaccine be used among this population[.]”)
May 13 (on or about).
CDC appears to have received a 13-page Pfizer Summary Monthly Safety Report for reporting period April 1, 2021, through April 29, 2021, titled “Pfizer-BioNTech COVID-19 Vaccine Myocarditis and Pericarditis.” (CDC FOIA 23-00756 CDC responsive records.pdf – Google Drive, p. 426), all of which is redacted except the cover page.
Amanda Cohn, MD, (Amanda Cohn | Biographies | About Us | NCBDDD | CDC), in an email with “Adolescent vaccine update” in the subject line, stated she spoke with Peter Marks | FDA, who “agreed communications around this is incredibly challenging and this is not the same as TTS [thrombosis with thrombocytopenia syndrome].” (CDC FOIA 23-00756 CDC responsive records.pdf – Google Drive, p. 268). While much of the content in the chain in which Dr. Cohn sent her email is redacted, and the word myocarditis does not appear in the unredacted part of the chain, it is reasonable to surmise the discussion was at least in part about myocarditis, in the context of discussion about COVID-19 vaccines for adolescents, because the FOIA production in which that record was provided was responsive to a request for CDC emails between certain personnel containing only one word (myocarditis). There are numerous such FOIA records which presumably have “myocarditis” redacted.
May 14.
CDC personnel receive an email from Jane Newburger, MD, MPH, Associate Cardiologist-in-Chief for Academic Affairs at Children’s Hospital Boston, which we quote almost in full: “As we anticipated, the post-vaccine myocarditis events have risen to the attention of our leaders at our institution (this is also a topic of conversation among Chief Safey Officers at Children’s hospitals). Mary Beth [Son] and I were emailed today by our hospital Emergency Management, stating Our hospital is putting together messaging for clinicians to provide information on myocarditis in COVID-vaccinated individuals and give direction on what to do if they think their patient is a case and asking for direction about to whom children should be referred.” “Do you think CDC will be having any sort of official statement about this soon? Or will it have to wait till analysis of cases? This is just for information for our hospital officials—I realize it is very unlikely for you to have a statement so soon.” (CDC FOIA 23-00756 CDC responsive records.pdf – Google Drive, p. 196.) (italics in original). One recipient was Dr. Cohn, who forwarded it to Dr. Walensky, stating: “Rochelle-I have a conflict with the 5 pm call about this issue, but I wanted to share the below note from Jane Newburger, given you[r] connection to BCH [emoji omitted]. This is part of the reason I think we need to say something about this issue.” (CDC FOIA 23-00756 CDC responsive records.pdf – Google Drive, p. 196). To which Dr. Walensky responded: “Got it…I just tried Dana, she is going to call me back in 5 min. For sure… R[.]” (p. 196) That was preceded by a May 13 email from Dr. Cohn to Dr. Newberger, stating in part: “We spoke to Manish [Patel] about the reports of myocarditis and MIS [multisystem inflammatory syndrome] like syndromes after vaccination in adolescents. I know the vaccine safety team at CDC is aware and working on setting up CISA [Clinical Immunization Safety Assessment] calls, but Sara [Mbaeyi] and I want to make sure we are on top of this from the program and communications perspective.” (p. 198) (hyperlinks added). Then, she asks for a meeting. (p. 198).
Kristen Nordlund, a CDC spokeswoman, received an 8:34 PM email from a general CDC mailbox (nipgrant@cdc.com), forwarded up to Dr. Walensky by 8:37 PM, with the subject line “For Awareness: Monitoring Reports of Myocarditis[,]” stating in part: “In recent weeks, there have been reports of myocarditis occurring after COVID-19 vaccination, including in Europe, where the EMA [European Medicines Agency] recently requested data from Pfizer and Moderna on reports of myocarditis and pericarditis after vaccination. CDC is aware of these reports, which are rare given the number of vaccine doses administered, and continues to monitor available data.” (p. 271). Continuing: “[W]e have been closely monitoring myocarditis/pericarditis in multiple safety systems, including [VAERS] and [VSD]. To date, there has not been a safety signal identified in VAERS or VSD.” “Healthcare providers should consider myocarditis in an evaluation of chest pain after vaccination and report all cases to VAERS[.]” (p. 271) (italics added). (On May 24, Dana Ohannessian, likely of the Massachusetts Department of Health, emailed Dr. Walensky what appears to be identical content.) (p. 311).
Dr. Su emailed multiple recipients with “Reports re myocarditis” in the subject line a (now redacted) graph showing reports of myopericarditis identified in VAERS by age group, stating: “Please be aware that these case reports are the result of an automated search, and include[] reports among people 18 years and younger that are currently under review[,]” forwarded to Dr. Walensky later that day. (p. 273). As what appears to be part of that email chain, Dr. Shimabukuro, stated: “We can always provide automated counts. They are informative in the sense that they give you the high end of the possible case reports. To date, we have not observed anything unusual or unexpected.” (p. 274) (italics added). Preceding that, in another email, he stated: “We have been monitoring myocarditis/pericarditis as a VAERS adverse event of special interest from the beginning.” (p. 275)
As the exchange between Drs. Brooks and Oster continues, Oster: “Don’t think it came up with ACIP [CDC Advisory Committee on Immunization Practices], but this topic [about five lines of text redacted] I think CDC needs to get ahead of this.” (p. 110). Brooks’ response: “[M]yocarditis in young men after mRNA vaccination is a top area of active investigation by CDC and FDA. Folks are reviewing VAERS and other data sources and collecting case reports.” (p. 109). We observe “myocarditis in young men after mRNA vaccination [being] a top area of active investigation” did not stand in the way of CDC’s recommending Pfizer’s COVID-19 vaccine for adolescents aged 12 and older two days earlier. Dr. Brooks continues (in frustration? mocking the situation?): “Although Israel, the European Medicines Agency, and US DoD are all also investigating the issue at present, a clear association has not yet been established. As such, the topic was not included in discussions during the recent ACIP meeting.” (p. 109) (italics added). The ACIP meeting immediately preceding May 14 was held May 5. (ACIP Meeting Slides| CDC). In other words, notwithstanding the safety signal in VAERS, the Australian and IMOH emails, IMOH’s likely having shared its data with CDC, all the dialog above, and talking points about myocarditis being prepared for Dr. Walensky on April 28—including about what was happening at DoD and in Israel—notwithstanding all that, the topic of myocarditis did not make it onto the May 5 ACIP meeting agenda, and seven days later CDC recommended use of Pfizer’s COVID-19 vaccine in adolescents ages 12 and older.
May 15.
The DIRECTOR’S BRIEF 20210516.pdf, emailed to Dr. Walensky late in the evening, contains the content which Kristen Nordlund sent her the previous day.
May 20.
Céline Gounder, while working on an op-ed for publication in the New York Times, emailed Drs. Walensky and Brooks, stating: “We’re hearing more reports of post-vaccine myocarditis from MA and NY…as well as elsewhere in the country. Is there anything you can share about post-vaccination myocarditis cases (demographics? fatal / non-fatal?) Or other serious post-vaccination events?” Dr. Gounder concludes, “These data could be just shared with us on background. We just want to make sure that in framing our argument, we’re not misrepresenting the facts.” (CDC FOIA 23-00756 CDC responsive records.pdf – Google Drive, p. 180).

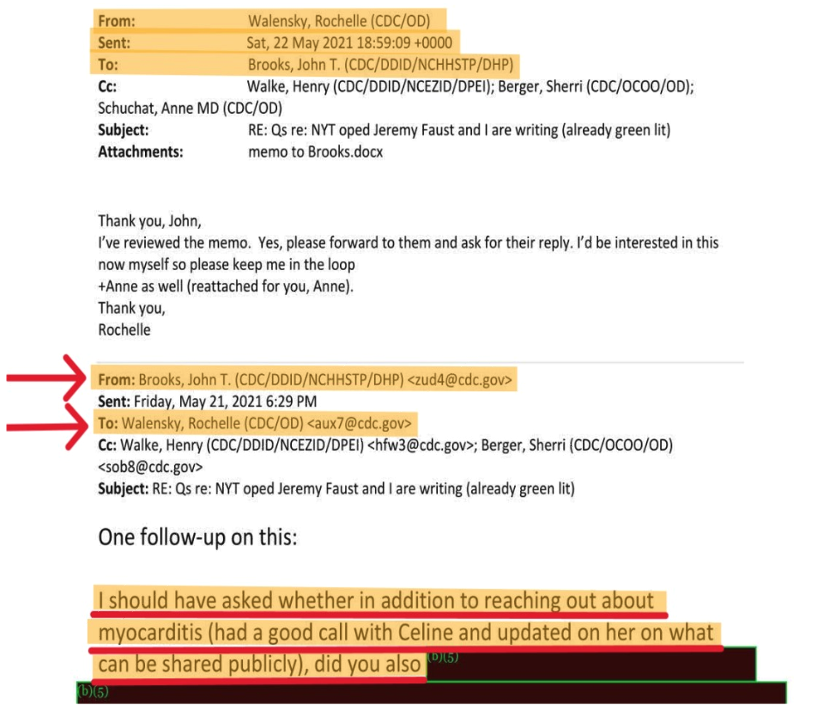
May 21.
Dr. Brooks emailed Dr. Walensky: “I should have asked whether in addition to reaching out about myocarditis (had a good call with Celine [Grounder] and updated [] her on what can be shared publicly)[,]” (p. 179) (italics added) to which Dr. Walensky responded on May 22, stating in part: “I’ve reviewed the [unspecified] memo. Yes, please forward to them and ask for their reply. I’d be interested in this now myself so please keep me in the loop[.]” (p. 179) (italics added). While speculation is exactly that, considering the May 22 and 23 emails Dr. Walensky received from Pfizer, discussed below, her statement—”I’d be interested in this myself now”—may indicate a growing disinclination to rely only on CDC subordinates for summaries of myocarditis incidence information, and instead consider the substantive information for herself. Dr. Walensky also received a substantive article about myocarditis on May 30 (see further below).
While this entire chronology reveals a heretofore non-public history of CDC’s internal discussions about myocarditis, about which the general public was unaware, Brooks’ statement about “updat[ing] Grounder] on what can be shared publicly” about myocarditis is the second of at least three acknowledgments that there were two sets of information about COVID-19 vaccines and myocarditis: what was discussed internally at CDC, and what could be shared publicly.
By 8:15 AM Eastern Time, CDC believed Washington State’s Department of Health was about to issue a “HAN alert” (“CDC’s primary method of sharing cleared information about urgent public health incidents with public information officers; federal, state, territorial, tribal, and local public health practitioners; clinicians; and public health laboratories.”) (Health Alert Network (HAN) | CDC), which made it up the organizational chart to Dr. Walensky by 10:03 AM. (CDC FOIA 23-00756 CDC responsive records.pdf – Google Drive, pp. 250-251). While the subject of the HAN alert is redacted, the FOIA production in which that record was provided was responsive to a request for CDC emails between certain personnel containing only one word (myocarditis), so it is reasonable to surmise Washington State’s contemplated HAN alert was about myocarditis.
May 22.
Dr. Walensky was forwarded, from a sender named Larry whose surname is redacted, what is likely a Pfizer document, titled “APPENDIX 3.7 SAFETY EVALUATION OF MYOCARDITIS AND PERICARDITIS.pdf.” (p. 424). She was sent that directly with no one copied. The original sender was Pfizer’s Patrick Caubel, Chief Safety Officer. His email reads: “This is the data for MYOCARDITIS (excluding pericarditis). Cut-off date is today.” (p. 424). Redactions follow, then: “Number of valid Adverse Events cases reported to Pfizer as of today is [redacted] are myocarditis cases.” (p. 424). More redactions follow, then: “Attached [is] our latest monthly aggregate analysis[,]” from January through May 2021 for 21 countries, with the number of cases now-redacted chart. (pp. 424-425). Larry’s forwarding email reads: “The attachment has the Israeli data included. It has all be sent to your team.” (p. 424).

May 23.
Dr. Walensky was updated about “Latest HAN” in an email with an attachment titled “DRAFT_Myocarditis_Advisory_05232021_1109” (p. 315). She asks: “Will it be full HAN?” This HAN is almost certainly CDC’s forthcoming HAN, separate from Washington State’s contemplated HAN. May 26 emails between CDC and HHS personnel with “HAN” in the subject line, and content like “[t]he initial draft is with Rochelle now[,]” support that the HAN under discussion was CDC’s, likely the forthcoming May 27 announcement. (https://drive.google.com/file/d/1ui6SpiVSTuNkgmJIAOT6TVrZWKS4QV4V/view?usp=share_link, pp. 23-26).
Dr. Walensky received a forwarded Pfizer email from a now-redacted sender, with no one copied, with “Myocarditis/age groups” in the subject line, the content of which was “Top graph is Myocarditis US ONLY, bottom is Myocarditis cumulative (all countries of occurrence).” (CDC FOIA 23-00756 CDC responsive records.pdf – Google Drive, p. 62). Those graphs are redacted in the FOIA records.
Dr. Walensky received an email from within CDC with attached PowerPoint slides titled “Myocarditis_update deck 2532021_FINAL (002)_hw.pptx[.]” (p. 318). Also, Dr. Walensky received a draft titled: “DRAFT_Myocarditis_Advisory_05232021_1109[.]” (p. 315).
In a staggering email, with “HAN on myocarditis” in the subject line, in a chain with “[w]e just got a heads up from FDA that CDC is issuing a HAN on myocarditis/mRNA vaccines[,]” written by Sarah Despres of HHS, Dr. Schuchat states: “Will follow up but believe aim [for issuing HAN] was for early this week. Just targeting Emergency departments rather than broad advisory.” (https://drive.google.com/file/d/1ui6SpiVSTuNkgmJIAOT6TVrZWKS4QV4V/view?usp=share_link, p. 128) (italics added). This is the third acknowledgment that there were two sets of information about COVID-19 vaccines and myocarditis: what was discussed internally at CDC, which in this instance was contemplated to be shared with emergency departments, but not broadly, presumably meaning not with the public, who were the targets on the national vaccination campaign.
May 24.
Some emails for talking points with Dr. Walensky’s May 25 Governor’s Call include an attachment titled: “Reactive on myocarditis and Tough QA_05232021 Mdocs” (https://drive.google.com/file/d/1ui6SpiVSTuNkgmJIAOT6TVrZWKS4QV4V/view?usp=share_link, p. 22).
Ms. Tumpey emailed Dr. Walensky, with Sherri Berger and Robert Goldstein, MD, Paul Fulton, and Jason McDonald, CDC Spokesperson, CCed, with “Draft WH [White House] Script and Slides” in the subject line, stating the attachment was the “draft press conf script and slides for your review.” (https://drive.google.com/file/d/1wgr-jdUTvvc8MgPnnghjcYLX1uC3L_tJ/view?usp=share_link, p. 18). Editing dialog follows.
May 25.
As part of that continuing email chain, Tumpey emailed Dr. Walensky two attachments: “CDC WH COVID Briefing TPs for 05252021_v4.docx[,]” and “CDC COVID WH Press Briefing 20210525.pptx[,]” stating, “Attached is the script with your edit and some tough Q/A at the end [redaction.] Is there anything else you need?” Editing dialog follows.
Benjamin Wakana, White House Deputy Director of Strategic Communications and Engagement, sent an email with “COVID Tough QA” in the subject line to Dr. Walensky; Anthony Fauci, MD; Andrew Slavitt, White House Senior Advisor for the COVID Response; Francis Collins, MD, PhD, Director, National Institutes of Health; Vice Admiral Vivek Murthy, MD, MBA, U.S. Surgeon General; and Marcella Nunez-Smith, MD, COVID-19 Health Equity Task Force Chair, CCing many others, mostly affiliated with the White House. The email contains an attachment titled, “Tough QA 5.24.21 11PM.docx” and says, “Hi, attached please find the latest tough QA. New topics include:”. The topics are redacted. The attached document, which appears to be 17 pages long, is fully redacted. (https://drive.google.com/file/d/1wgr-jdUTvvc8MgPnnghjcYLX1uC3L_tJ/view, pp. 28-45).
May 27.
Ms. Nordlund sent a high-importance email to Stephanie Caccomo and Lorrie McNeill, both at FDA, CCing CDC personnel, with “Updated myocarditis documents” in the subject line, “Thank[ing] [them] for jumping on the phone with us earlier this afternoon to talk through the language around aftercare.” (CDC FOIA 23-00756 CDC responsive records.pdf – Google Drive, p. 287). The remaining one-fifth of a page of content is redacted. (p. 287).
May 28.
In the context of CDC-HHS discussions about an article in AAP News, the American Academy of Pediatrics official news magazine, titled “Pediatricians should report cases of heart inflammation after COVID-19 vaccination” (https://drive.google.com/file/d/1ui6SpiVSTuNkgmJIAOT6TVrZWKS4QV4V/view?usp=share_link p. 36), Dr. Walensky emailed Dr. Rachel Levine at HHS, copying two others, saying: “So, I’m deep in the weeds (and perhaps too much so) but I’d like to understand how they are thinking about this.” (p. 85).
Grace Kwak, White House Advisor to the Deputy COVID-19 Response Coordinator, Executive Office of the President, asks Dr. Walensky’s Executive Assistant, Lynn Gershman, where to find “papers/briefings” about “updates on TTS [thrombotic thrombocytopenia syndrome], myocarditis, etc.” (https://drive.google.com/file/d/1im9ulZAPs3aalBsT2HbxnMu8TlRmP7y-/view?usp=share_link, 46). Ms. Gersham forwarded the “request from WH” to Ms. Berger. (p. 46).
Late May.
While the above intra- and inter-agency dialog about myocarditis was happening, the general public’s interest about myocarditis from COVID-19 vaccines was soaring. The black jagged line on the graph below represents Google searches for “COVID vaccine myocarditis” around this time.
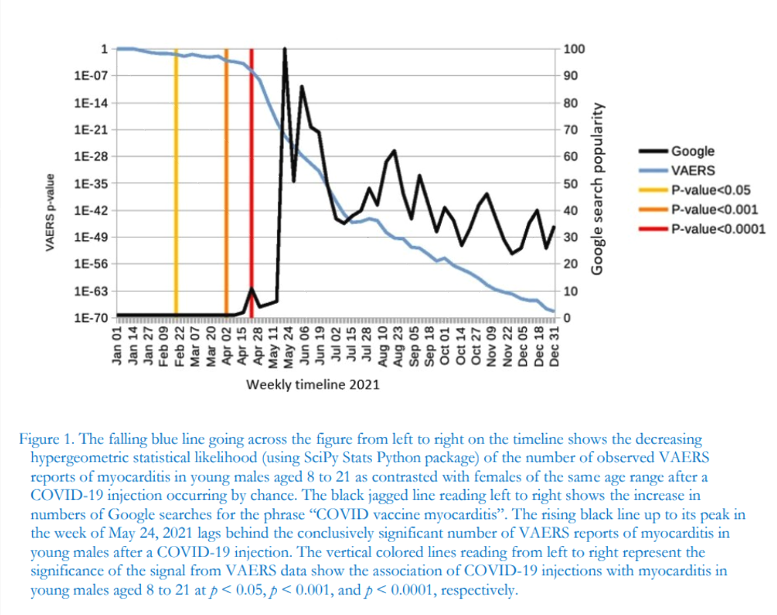
Jablonowski, K., & Hooker, B. S. (2022). Delayed Vigilance: A Comment on Myocarditis in Association with the COVID-19 Injections. International Journal of Vaccine Theory, Practice, and Research, 2(2), 651.1–651.4. https://doi.org/10.56098/ijvtpr.v2i2.61; https://tinyurl.com/2p9f523n (used with permission).
May 27 or 29.
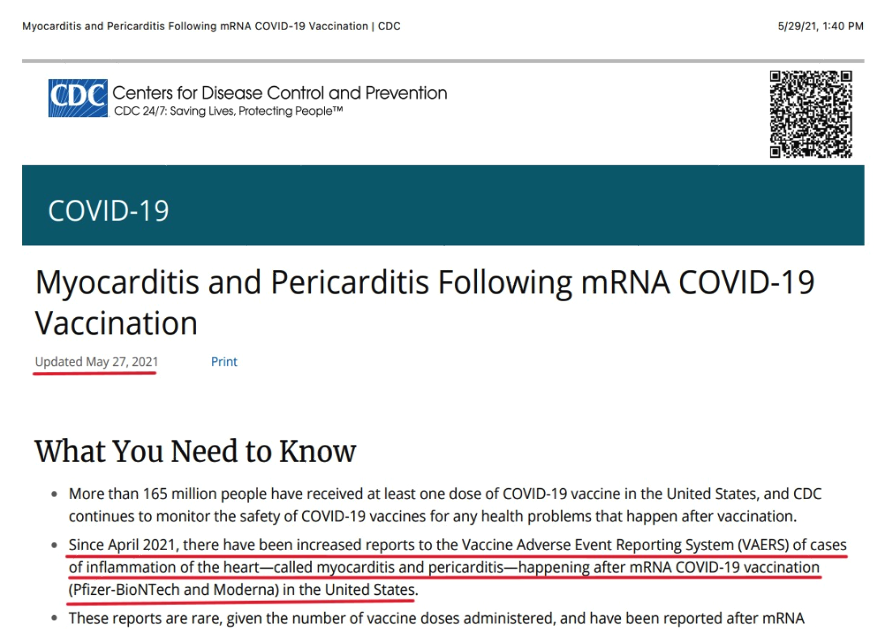
Finally, CDC disclosed post-vaccination myocarditis and pericarditis: “Since April 2021, there have been increased reports to the Vaccine Adverse Events Reporting System (VAERS) of cases of inflammation of the heart—called myocarditis and pericarditis—happening after mRNA COVID-19 vaccination (Pfizer-BioNTech and Moderna) in the United States[,]” and: “These reports are rare, given the number of vaccine doses administered[.]” (Myocarditis and pericarditis following mRNA COVID-19 vaccination (cdc.gov); https://tinyurl.com/5b9ratwn). It is unclear whether CDC’s belated disclosure of the myocarditis risk was made to the public on May 27 or May 29. The announcement has both dates on it: “Updated May 27, 2021” (middle left below) and “5/29/21, 1:40 PM” (top right below).
Closely parsed, “increased reports” can be read to mean there have been reports of myocarditis and pericarditis throughout the COVID-19 vaccination rollout, but since April, the number of reports has increased.
Whether “[t]hese reports are rare[,]” has been debated. Rare or not, the graph below provides context, by comparing incidence of myocarditis occurring after non-COVID-19 vaccinations since the inception of VAERS in the 1990s, with incidence of myocarditis occurring after COVID-19 vaccination. Placed in that context, myocarditis and pericarditis incidence after COVID-19 vaccination is anything but rare.
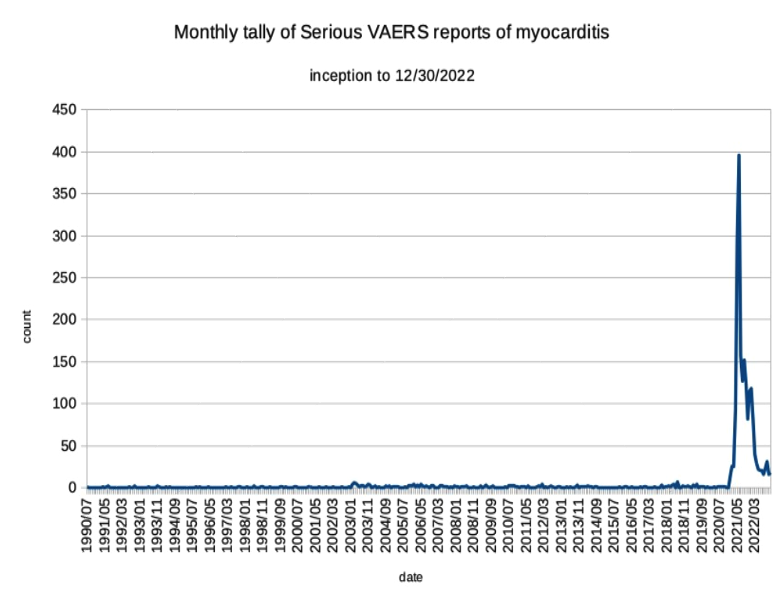
Graph prepared by Karl Jablonowski, PhD. (used with permission).
May 29.
Dr. Goldstein emailed Dr. Walensky a myocarditis-related article. (Prevalence of Clinical and Subclinical Myocarditis in Competitive Athletes With Recent SARS-CoV-2 Infection: Results From the Big Ten COVID-19 Cardiac Registry | Cardiology | JAMA Cardiology | JAMA Network, https://tinyurl.com/3w58cftb) (CDC FOIA 23-00756 CDC responsive records.pdf – Google Drive, pp. 20-30).
While this article was being written, further CDC FOIA 23-00756 documents were produced, which DailyClout posted and discussed here: FOIA’d Emails Reveal Highest-Level Leaders at White House, HHS, CDC, NIAID, AAP All Knew COVID Vaccines Linked to Myocarditis, Yet Publicly Covered Up Findings. – DailyClout. Herein, we have drawn minimally from that production.
Having provided that chronology with little comment, our planned second article will explore the prospects for state-level investigation and prosecution, if supported, including discussing which government entities could investigate and in what forums, who could be investigated and for what possible offenses (including reckless endangerment and official misconduct), federal supremacy clause immunity, jurisdiction and venue, and statute of limitations and tolling.
While we will develop our discussion there, we make one observation here. While CDC internally was discussing communications strategies, pressers, talking points, being on top things from a communications perspective, regarding information about myocarditis, some of which was “not for public release” but for “awareness,” or divided into parts that “can be shared publicly” and not, or only for emergency departments (where some COVID-19 vaccinees would go to after they made their uninformed decision), Americans—using Jablonowski and Hooker’s 112,000,000 figure—were lining up to get vaccinated at rate of over 1.1 million per day, every day, during the identified period, exposing themselves to a myocarditis risk “the nation’s leading science-based, data-driven, service organization that protects the public’s health[,]” (About CDC | About | CDC; https://tinyurl.com/39k5s5zm), which uses “the Vaccine Adverse Event Reporting System (VAERS) [as] a national early warning system to detect possible safety problems in U.S.-licensed vaccines” (VAERS – About Us (hhs.gov); https://tinyurl.com/ythdxxth), had been discussing internally, for months, but did not say anything about publicly until May 27. Further, because of the heavily redacted FOIA productions, we do not know the full scope of what CDC internally discussed about myocarditis during those months in our history.


