Report 40: Data Do Not Support Safety of mRNA COVID Vaccination for Pregnant Women


Commentary on “Preliminary Findings of mRNA Covid Vaccine Safety in Pregnant Persons” as Reported by the Centers for Disease Control and Prevention and the Food and Drug Administration, June 17, 2021, New England Journal of Medicine
Note: On September 26, 2022, Dr. Chandler published a follow-up report to the report below. The follow-up report, Report 40: “2021 CDC and FDA Misinformation – Retroactive Editing, Erroneous Spontaneous Abortion Rate Calculation, Obfuscation in the New England Journal of Medicine”, provides in-depth analysis of how an allegedly prestigious medical journal played a shell game with data and information so that its readers could not accurately determine if there were data to support the safety of mRNA COVID vaccination in pregnant women. After a mind-boggling journey through “corrections,” “updates,” and multiple issues of NEJM, no such data exists.
Currently, the Centers for Disease Control and Prevention (CDC), Advisory Committee on Immunization Practices (ACIP), American College of Obstetricians and Gynecologists (ACOG), and the American Academy of Pediatrics (AAP) recommend that Covid-19 vaccines should not be withheld from pregnant women. The following analysis will show that no accurate, reliable scientific data were collected; and, thus, it is not possible to provide useful information about the risks to pregnant women and their babies from Covid mRNA vaccines. Because of this, medical and public health organizations are remiss in their duties to protect the health and well-being of patients when they endorse the use of mRNA vaccines in pregnant women. [https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html, https://www.cdc.gov/vaccines/hcp/acip-recs/rec-vac-preg.html, https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care, and https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/covid-19-vaccine-for-children/about-the-covid-19-vaccine-frequently-asked-questions/.]
I. Context:
This article was undertaken as part of a widespread review of Food and Drug Administration (FDA)-released Pfizer documents concerning their experimental lipid nanoparticle plus messenger ribonucleic acid gene (LNP/mRNA) therapy drug, BNT162b2.
On January 6, 2022, Judge Mark T. Pittman of the United States District Court in the Northern District of Texas ordered the release of the Pfizer clinical trial documents. [https://www.sirillp.com/wp-content/uploads/2022/01/ORDER_2022_01_06] The FDA had requested that the documents be sealed for 75 years.
Pfizer completed Phase 3 trials of BNT162b2 in fall of 2020 and submitted its application for an Emergency Use Authorization (EUA) to the FDA on November 20, 2020. On December 11, 2020, the FDA issued an Emergency Use Authorization (EUA). Widespread distribution and mass inoculation began shortly afterward. [https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19]
Moderna received approval for their product, mRNA-1273, along a similar timeline. [https://www.aha.org/2020-12-19-special-bulletin-summary-fda-emergency-use-authorization-modernas-covid-19-vaccine]
The New England Journal of Medicine (NEJM) published a research article, Shimabukuro, et. al., on June 17, 2021, (online publication) authored by 21 affiliates of the CDC and FDA on behalf of the 47-member CDC and FDA Pregnancy Registry Team entitled “Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons.” [https://www.nejm.org/doi/full/10.1056/NEJMoa2104983] NEJM first published this on April 21, 2021, and updated it on September 8, 2021. However, the April and September versions are not available online, despite NEJM stating, “This article was published on April 21, 2021, and updated on September 8, 2021, at NEJM.org.” [https://www.nejm.org/doi/full/10.1056/NEJMoa2104983]
This study reported results of 35,691 pregnant women, entered into the V-safe Registry maintained by the CDC, who received at least one dose of either the Pfizer/BioNTech or Moderna Covid-19 drug during the ten-week period from December 14, 2020, through February 28, 2021. [https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafepregnancyregistry.html]
Results from the Vaccine Adverse Event Reporting System (VAERS) were also queried, and the results are presented.
Shimabukuro, et. al. identify the then and now current CDC policy regarding use of new SARS-CoV-2 Spike encoding genetic products from Pfizer and Moderna in pregnant women:
The Centers for Disease Control and Prevention (CDC) and ACIP, in collaboration with the American College of Obstetricians and Gynecologists and the American Academy of Pediatrics, have issued guidance indicating that Covid-19 vaccines should not be withheld from pregnant persons. Italics added.
[https://www.nejm.org/doi/pdf/10.1056/NEJMoa2104983?articleTools=true, p. 2274, and https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care.]
This article focuses on the basis of this recommendation in light of the CDC and FDA documentation presented in Shimabukuro, et. al.
II: Registry Data and Data Mining
A registry, one of the scientifically weakest clinical research tools, ranks far below the gold standard randomized, double-blinded, tightly controlled study of at least two years duration or a prospective tightly controlled matched subject study.
SAP, a competitor to SAS the well-regarded maker of the statistical package used by the CDC and FDA on this project, notes the following about data mining:
“With masses of new data, there are also masses of incomplete, incorrect, misleading, fraudulent, damaged, or just plain useless data. The tools can help sort this all out, but the users must be continually aware of the source of the data and its credibility and reliability.” [https://www.sap.com/insights/what-is-data-mining.html]
Data professionals often refer to this as “GIGO” or “garbage in, garbage out.” A registry can be used to detect signals, but it certainly does not generate robust, high-quality scientific data.
III. Methodology
Study samples reported in the Shimabukuro, et. al. report came from two databases.
#1: The V-safe Surveillance System and Pregnancy Registry is a voluntary, smartphone-based surveillance system maintained by the CDC. Participants agree to receive periodic emails to which they respond. [https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafepregnancyregistry.html]
One estimate puts the rate of participation in V-safe at about five percent of all those given the LNP/mRNA drug. [V-safe COVID-19 vaccine pregnancy registry. Atlanta: Centers for Disease Control and Prevention. May 3, 2021.
(https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafepregnancyregistry.html).]
From V-safe, participants were contacted and entered into a second registry, the Pregnancy Registry, from which data for this report were drawn. Those managing the Registry planned to collect data for 12 months.
#2: The second registry used was the Vaccine Adverse Event Reporting System (VAERS), a voluntary reporting system maintained by the CDC and FDA that was established 30 years ago to monitor side effects of vaccines. [https://vaers.hhs.gov/] The CDC verifies VAERS entries.
Diversity of opinion exists as to what percentage of actual adverse events (AEs) the VAERS reporting comprises – from a single-digit Under Reporting Factor (URF) of one to upwards of 40-plus times. Overreporting is less likely given the verification process. The reader should keep this URF range of 1 to over 40 in mind for any VAERS data.
[https://vaersanalysis.info/2021/12/13/using-cms-whistleblower-data-to-approximate-the-under-reporting-factor-for-vaers/ and https://digital.ahrq.gov/sites/default/files/docs/publication/r18hs017045-lazarus-final-report-2011.pdf]
This article will also make reference to a third database maintained by Pfizer as reported in “Confidential Document 5.3.6.” [https://robertchandler.substack.com/p/pfizer-document-536-cumulative-analysis]
A. V-safe/Pregnancy Registry Data
Outcomes were assessed in terms of comparison of reactogenicity in pregnant and non-pregnant women aged 16-54 years, as well as pregnancy outcomes.
Reactogenicity is a concept applied to vaccines and includes early reaction to drug products such as pain at the injection site, fever, other short-term signs and symptoms. [https://pubmed.ncbi.nlm.nih.gov/31583123/]
Reactogenicity differs from Adverse Events (AEs) and Adverse Events of Special Interest (AESI), which focus on specific categories of events and specific diagnoses by function and or organ system.
Pregnancy outcomes (Shimabukuro, et. al., https://www.nejm.org/doi/pdf/10.1056/NEJMoa2104983?articleTools=true, p. 2275.) in the Pregnancy Registry were assessed in a subset of completed pregnancies in terms of spontaneous abortion (loss of fetus in the first 20 weeks, also called ‘miscarriage’), stillbirth (loss of fetus after 20 weeks), pre-term birth, congenital anomalies, small size for gestational age and neonatal death.
Multiple factors cause the loss of a fetus, and such loss occurs decreasingly as a function of duration of gestation. The miscarriage rate is highest in the first six weeks, and most fetal loss occurs in the first trimester.
[https://obgynkey.com/chapter-6-first-trimester-abortion/https://my.clevelandclinic.org/health/diseases/9688-miscarriage#diagnosis-and-tests Journal of Epidemiology and Community Health 38(2):143-8] [https://www.nejm.org/doi/10.1056/NEJM198807283190401?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed]
Data are entered and database queries return results from the system. Each query can return data from different subjects. It must be understood that the numbers returned from each query likely represent a unique set of subjects making comparisons across queries problematic.
Contrast this process with a cohort study (one that takes in a predetermined number of subjects and actively follows them prospectively to completion) that produces a complete data set for all or most of those enrolled in the study. Data from this more robust type of study are very limited.
B. VAERS Data
“VAERS is a passive reporting system, meaning it relies on individuals to send in reports of their experiences to CDC and FDA. VAERS is not designed to determine if a vaccine caused a health problem, but is especially useful for detecting unusual or unexpected patterns of adverse event reporting that might indicate a possible safety problem with a vaccine. This way, VAERS can provide CDC and FDA with valuable information that additional work and evaluation is necessary to further assess a possible safety concern.” [https://vaers.hhs.gov/about.html]
Analysis of VAERS reporting included Adverse Events (AEs) that are both pregnancy and non-pregnancy related.
IV. Outcomes:
There were 35,691 pregnant women entered into CDC’s V-safe COVID-19 Pregnancy Registry system during the first two and a half months after EUA. Remember that these almost 36,000 pregnant women may be just five percent of the total number of pregnant women injected with the LNP/mRNA drug as of February 28, 2021. If they represent only five percent, then the real total of pregnant women who received the drug would be close to 720,000.
Of the 35,691 cases 5,230 were contacted and offered enrollment in the Pregnancy Registry and a total of 3,958 agreed and qualified for further study.
Table 1: V-Safe Data Set
| As of 3/30/2021 for data 12/14/2020 thru 2/28/2021
|
||
| From Table 1, Shimabukuro, et. al.3 | ||
| mRNA + Pregnancy Cases | 35691 | |
| Pregnant at time of Injection | 30887 | |
| + Pregnancy test after injection | 4804 | |
Table 2 gives the summary statistics for the Pregnancy Registry.
Table 2: Pregnancy Registry
| Pregnant at or shortly after Injection | 5230 |
| Unreachable | 912 |
| Declined | 86 |
| Did not meet inclusion criteria | 274 |
| Eliminated | 1272 |
| Net | 3958 |
These 3,958 pregnant women were the subjects of further analysis, but here is where the caution from SAS applies. The numbers reported in each category may refer to results of a data query rather than unique individuals followed through various cuts. This is important in coming to an understanding of what exactly is being reported in Shimabukuro, et. al.
A. Spontaneous Abortion Rate (SABR)
Spontaneous abortion does not include medically induced loss of fetus or stillbirths. [https://www.emedicinehealth.com/what_are_abortion_and_miscarriage/article_em.htm]
Of the 3,958 pregnant women entered into the V-safe Pregnancy database, 1,132 cases received LNP/mRNA drugs during their first trimester and another 1,714 in their second trimester totaling 2,846 subjects injected during the first 24 weeks after conception, per Table 3. [https://www.nejm.org/doi/pdf/10.1056/NEJMoa2104983?articleTools=true, p. 2279.]
There were 2,846 combined first and second trimester subjects representing seventy-two percent of those receiving LNP/mRNA during pregnancy who were entered into the Pregnancy Registry and seven percent of the entire sample of 35,691 pregnant women receiving LNP/BNT162b2. From authors’ Table 3, page 2279 [https://www.nejm.org/doi/pdf/10.1056/NEJMoa2104983?articleTools=true]:
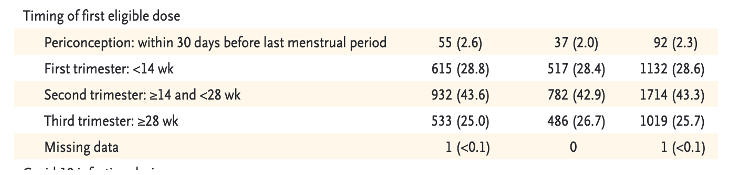
Only 827 subjects out of the 3,958 cases enrolled in the Pregnancy Registry completed pregnancy during the study period. Random selection cannot be assumed based on information provided.
This represents twenty-one percent of the group entered into the pregnancy registry and 2.3% of the initial group of 35,691 pregnant women drawn from the total pool. 827 represents about 0.11% of the estimated total number of pregnant women injected with LNP/mRNA in the first 10 weeks following EUA.
The most profound change to the fetus occurs in the first trimester, and spontaneous abortion rates are much higher during this phase.
Therefore, pregnant women receiving LNP/mRNA during their first trimester are of special interest in terms of spontaneous abortion, prematurity, small size for gestational age, congenital anomalies, and neonatal death.
Caution: Multiple attempts have been made to calculate rates of spontaneous abortion from these data.
Four determinations of the rate of spontaneous abortion after LNP/mRNA treatment in pregnant women will be illustrated. A fifth, referred to as an MSU (Make Stuff Up) Analysis, will be addressed in a subsequent article.
1. V-safe Analysis 1:
Shimabukuro, et. al. reported on spontaneous abortions as follows:
“Among 827 participants who had a completed pregnancy, the pregnancy resulted in a live birth in 712 (86.1%), in a spontaneous abortion in 104 (12.6%), in stillbirth in 1 (0.1%), and in other outcomes (induced abortion and ectopic pregnancy) in 10 (1.2%). A total of 96 of 104 spontaneous abortions (92.3%) occurred before 13 weeks of gestation (Table 4), and 700 of 712 pregnancies that resulted in a live birth (98.3%) were among persons who received their first eligible vaccine dose in the third trimester.” [https://www.nejm.org/doi/pdf/10.1056/NEJMoa2104983?articleTools=true, p. 2276.]
Here the authors’ calculated a 12.6% rate of spontaneous abortion using 104 as the numerator and 827 as the denominator.
However, this is a gross error as spontaneous abortion refers to loss of the fetus during the first 20 weeks, and the 827 included 700 third trimester pregnancy cases. So, using 827 as a denominator is erroneous and misleading.
Later attempts were made to retroactively change this number, but it remains in the June 17, 2021, online version of the article. [https://www.nejm.org/doi/full/10.1056/NEJMoa2104983] A September 8, 2021, editorial effort successfully deleted the calculation from Table 4 of the June 17, 2021, version as acknowledged in the NEJM on October 14, 2021, but the 12.6% figure remains in the text. [https://www.nejm.org/doi/full/10.1056/NEJMc2113516]
Additionally, only 127 participants received LNP/mRNA products during the first and second trimesters. Why lump first and second trimester cases together? The risk for spontaneous abortions is almost all in the first trimester.
2. V-safe Analysis 2:
Some have attempted to pull the first trimester cases out of the data to match these cases with the 20-week abortion group. Why not match the 20-week group with the 20-week spontaneous abortions? Great question.
Here is how Analysis 2 goes.
Authors’ Table 4 reports 104 spontaneous abortions during the first 20 weeks. [https://www.nejm.org/doi/pdf/10.1056/NEJMoa2104983?articleTools=true, p. 2280.]
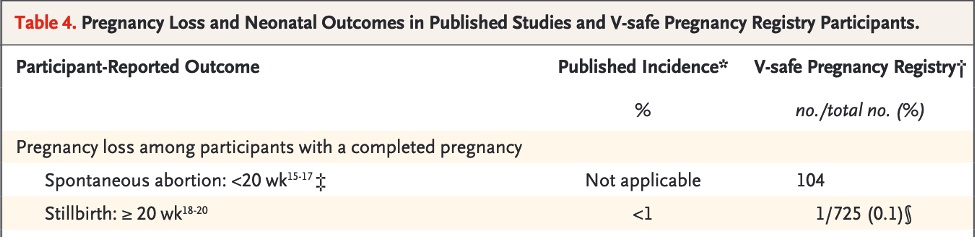
Table 2 below summarizes the data regarding the numbers of total pregnant women in V-safe, the number entered into the Pregnancy Registry and the number who complete their pregnancy.
Given as well is the number of spontaneous abortions in the first 20 weeks.
Table 2: CDC Spontaneous Abortions
[Reference James Thorp, M.D.’s calculations in the comments section of https://pierrekory.substack.com/p/massive-miscarriage-rates-among-vaccinated]
|
A spontaneous abortion rate of 82% appears to be impossibly high compared with published rates of 10-20%. [https://www.mayoclinic.org/diseases-conditions/pregnancy-loss-miscarriage/symptoms-causes/syc-20354298]
The 104 mothers with spontaneous abortions are probably not from the same query pool as the 127, making this calculation erroneous as well.
To date, the raw data have not been made available even though this paper was published 14 months ago. Independent analysis and verification are therefore impossible.
[https://www.nejm.org/doi/suppl/10.1056/NEJMoa2104983/suppl_file/nejmoa2104983_data-sharing.pdf]
3. V-safe Analysis 3:
This analysis begins with the completed pregnancies as the rest of the figures above 827 in Table 2, i.e., 3958 and 35691, are unchanged.
| Completed pregnancies | 827 |
| Live births | 712 |
| 1st and 2nd Trimester | 12 |
| 3rd Trimester | 700 |
| Spontaneous abortions + Stillbirth | 115 |
| Spontaneous abortions before 13 weeks gestational age | 96 |
| Pregnant woman injected within 30 days before the first day of the last menstrual period or in the first trimester the first day | 1224 |
| No follow up through 20 weeks | 905 |
| Follow up through 20 weeks | 319 |
| Spontaneous abortions @<20 weeks | 104 |
| Spontaneous abortions @<20 weeks | 33% |
Unfortunately, this approach falls victim to the same flaw as in Analysis 2, multiple unique groups.
4. V-safe Analysis 4:
If one waited until the October 2021 update to read this paper, he or she would have been rewarded with the final analysis as contained in this bizarre statement:
|
No denominator was available to calculate a risk estimate for spontaneous abortions because at the time of this report, follow-up through 20 weeks was not yet available for 905 of the 1224 participants vaccinated within 30 days before the first day of the last menstrual period or in the first trimester. Spontaneous abortions @<20 weeks Unknown |
[https://www.nejm.org/doi/full/10.1056/NEJMx210016]
Bottom line: Computation of spontaneous abortion rate from V-safe Registry data does not produce a reasonable estimate of the true rate of spontaneous abortion in women given LNP/mRNA products during pregnancy, particularly during the critical first 12 to 14 weeks.
5. VAERS Registry Spontaneous Abortion Rate
Perhaps VAERS can help? Table 5 shows the results of the VAERS database query. [https://vaers.hhs.gov/]
Table 5: VAERS
| Pregnant women | 221 |
| Non pregnancy AEs | 155 |
| Pregnancy/Neonatal AEs | 66 |
| Pregnancy related AEs | |
| Spontaneous abortions (SAs) | 46 |
| 1st Trimester SAs | 37 |
| 2nd Trimester SAs | 2 |
| Unknown | 7 |
| Stillbirth | 3 |
| Premature membrane rupture | 3 |
| Vaginal bleeding | 3 |
The authors do not provide the logic or terminology used to query the VAERS database making verification of these numbers impossible.
Unfortunately, not much can be concluded from this tiny, non-random sample of cases other than to note the potential harms of LNP/mRNA in pregnant women and their babies.
6. Pfizer Registry Abortion Rate
For comparison with V-safe and VAERS, there is Pfizer document “5.3.6 CUMULATIVE ANALYSIS OF POST-AUTHORIZATION ADVERSE EVENT REPORTS OF PF-07302048 (BNT162B2) RECEIVED THROUGH 28-FEB-2021” that reports Adverse Events in its own registry collected during the same time period covered by the CDC data and reports on spontaneous abortions in 28 completed pregnancies. [https://phmpt.org/wp-content/uploads/2022/04/reissue_5.3.6-postmarketing-experience.pdf and https://robertchandler.substack.com/p/why-do-females-have-more-adverse]
The trimester of the injection(s) was /were not given.
Altogether there were 270 pregnant women who received LNP/mRNA injections, but outcome was not known in 238 and 5 were in progress.
Table 3: Pfizer Spontaneous Abortions
|
Pregnancies with outcomes out of 270 PW |
28 | |
| Spontaneous abortion | 23 |
82% |
With 88% of the pregnant women unaccounted for and no information provided about injection date as a function of gestational age no reasonable estimate of spontaneous abortion rate can be made from these data.
Using the 720,000 estimate of the actual number of pregnant women receiving LNP/mRNA from the V-safe Registry in the first 10 weeks, these 28 cases represent a non-random sample of 0.004% of the estimated total number of pregnant women given experimental gene products during the period from December 14, 2020, to February 28, 2021.
B. Pre-Term, Small Size and Congenital Anomalies.
Of the 724 live-born infants in the V-safe registry there were 60 of 636 pre-term births, 23 of 724 small for gestational period and 16 of 724 with major congenital anomalies.
Table 4: Pre-Term, Small size and congenital anomalies.
|
Pre-Term Cases |
60/636 | 636 vax before 37 wks. |
| Small size for gestational period | 23/724 | 8% |
| Major congenital anomalies | 16/724 |
3% |
|
|
This data is virtually meaningless since there is no trimester data, no data about age at conception, comorbidities, number of prior pregnancies and births and so on.
C. Dose Related Reactogenicity
Shimabukuro, et. al. present the following reactogenicity data in their Table 2. [https://www.nejm.org/doi/pdf/10.1056/NEJMoa2104983?articleTools=true, p. 2277.]
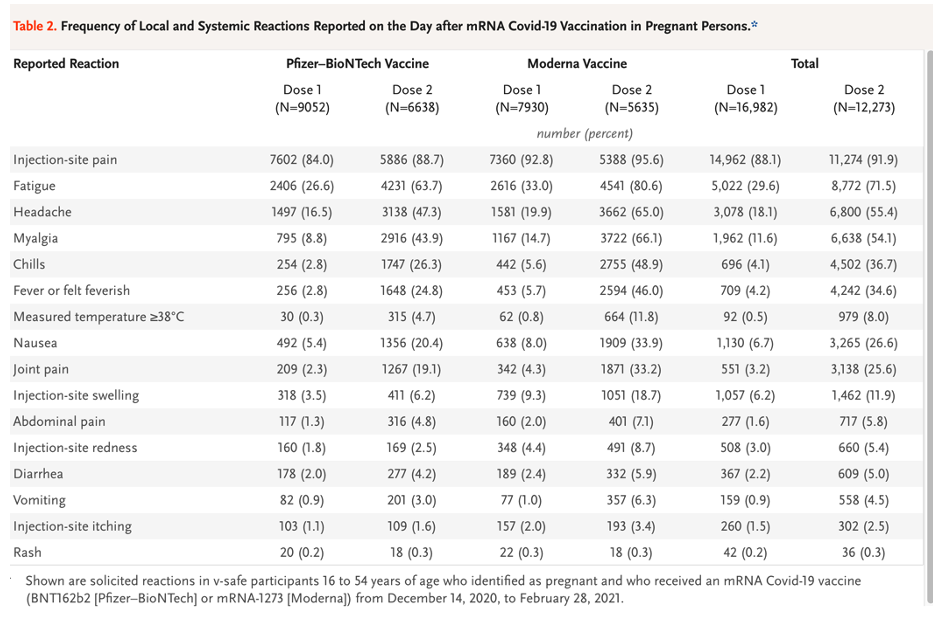
There may be four different data samples represented here, which is a typical finding in a data mining project.
Tests of statistical significance were not performed on this data, but there appears to be a dose-related effect here that reinforces the observation from Pfizer pre-clinical and clinical trials that there is a dose-related response to LNP/mRNA. Dose-related adverse events are events that increase in frequency as the total amount of drug received increases and are of concern when considering the possible cumulative frequency and severity of AEs and AESI rates in a multiple booster program.
V. Omissions
The CDC authors neglected to mention the relevant omissions from the preclinical studies as reported in Pfizer confidential document ,“2.4 NONCLINICAL OVERVIEW,” and listed below [https://phmpt.org/wp-content/uploads/2022/03/125742_S1_M2_24_nonclinical-overview.pdf and https://robertchandler.substack.com/]:
A. Pre-Clinical Studies:
- Safety pharmacology: “No safety pharmacology studies were conducted with BNT162b2 as they are not considered necessary for the development of vaccines according to the WHO guideline (WHO, 2005).” [https://phmpt.org/wp-content/uploads/2022/03/125742_S1_M2_24_nonclinical-overview.pdf, p.14,¶2]
- Pharmacodynamic Drug Interactions: “Nonclinical studies evaluating pharmacodynamic drug interactions with BNT162b2 were not conducted as they are not generally considered necessary to support development and licensure of vaccine products for infectious diseases (WHO, 2005).” [https://phmpt.org/wp-content/uploads/2022/03/125742_S1_M2_24_nonclinical-overview.pdf, p. 14, ¶3]
- No pharmacokinetic studies: were performed with BNT162b2 and “…are generally not considered necessary to support the development and licensure of vaccine products for infectious diseases (WHO, 2005, WHO, 2014).” [https://phmpt.org/wp-content/uploads/2022/03/125742_S1_M2_24_nonclinical-overview.pdf, p. 17, ¶1]
- “The protein encoded by the RNA in BNT162b2 is expected to be proteolytically degraded like other endogenous proteins. RNA is degraded by cellular RNases and subjected to nucleic acid metabolism. Nucleotide metabolism occurs continuously within the cell, with the nucleoside being degraded to waste products and excreted or recycled for nucleotide synthesis. Therefore, no RNA or protein metabolism or excretion studies will be conducted.” [https://phmpt.org/wp-content/uploads/2022/03/125742_S1_M2_24_nonclinical-overview.pdf, p. 20, ¶3]
- Genotoxicity: “No genotoxicity studies are planned for BNT162b2 as the components of the vaccine construct are lipids and RNA are not expected to have genotoxic potential (WHO 2005).” [https://phmpt.org/wp-content/uploads/2022/03/125742_S1_M2_24_nonclinical-overview.pdf, p. 29, ¶3]
- “Carcinogenicity studies with BNT162b2 have not been conducted as the components of the vaccine are lipids and RNA and are not expected to have carcinogenic or tumorigenic potential (WHO 2005).” [https://phmpt.org/wp-content/uploads/2022/03/125742_S1_M2_24_nonclinical-overview.pdf, p. 29, ¶4]
These omissions were not mentioned in the Shimabukuro, et. al. paper which was and continues to be used as reference for medical professionals charged with informing patients about the risks, benefits, and alternatives to never before used experimental gene therapy drugs that have the potential for gene modification, carcinogenesis, autoimmunity and a host of other medical problems both known and unknown.
It is now known that Spike proteins, mRNA and lipid nanoparticles are present for weeks to months, and possibly years, in human tissues, and the harms from these agents are being identified almost daily. [https://robertchandler.substack.com/p/bnt162b2-mrna-expresses-modified]
B. Biodistribution Data:
Another study not mentioned in the CDC document concerns the biodistribution of BNT162b2 that shows accelerating accumulation of LNP/mRNA in Wistar Han Rat ovaries, below Chart 4. [https://phmpt.org/wp-content/uploads/2022/03/125742_S1_M2_24_nonclinical-overview.pdf, pp. 15-20] We have no such data in humans.
Chart 4
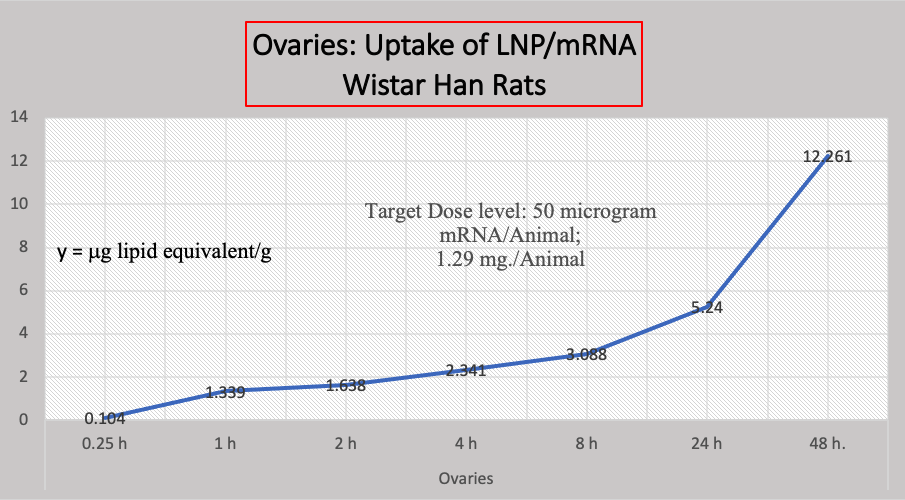
Criticisms here have been that the dose may have not been suitable, that these biodistribution studies should have been run longer than 48 hours, and that animal studies can give misleading or erroneous results.
So, is it not possible to compress ten years of novel drug development into ten months? The simple answer is, exactly.
C. Phase 3 Clinical Trials:
What about the large Phase 3 clinical trial reported by Polack, et. al.?
This report does not address the prevention of Covid-19 in other populations, such as younger adolescents, children, and pregnant women. Safety and immune response data from this trial after immunization of adolescents 12 to 15 years of age will be reported subsequently, and additional studies are planned to evaluate BNT162b2 in pregnant women, children younger than 12 years, and those in special risk groups, such as immunocompromised persons. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7745181/pdf/NEJMoa2034577.pdf, p. 12.]
Keep in mind that this was published on December 16, 2020, mass inoculation began December 14, 2020, and by February 28, 2021, at least 35,691 pregnant women had been given LNP/mRNA gene therapy products and these pregnant women and their babies was largely lost to follow up.
This is another point about the Phase 3 trial subjects. Volunteers in the Placebo group were offered, and many were given, LNP/mRNA drugs thus ending the randomized, controlled study that should have lasted at least two years.
This was the best shot at understanding the possible harms of LNP/mRNA.
D. Sex Differences in Adverse Events and Adverse Events of Special Interest.
Another major omission from the CDC report about safety using LNP/mRNA in pregnant women concerns the data from Pfizer summary report 5.3.6 that shows a strong signal of increased harms from the RNA drugs to women in general, as seen below in Chart 5. [For source data, reference https://www.phmpt.org/wp-content/uploads/2022/04/reissue_5.3.6-postmarketing-experience.pdf.]
Chart 5: Sex Difference
Pfizer Adverse Events 5.3.6
2/28/2021
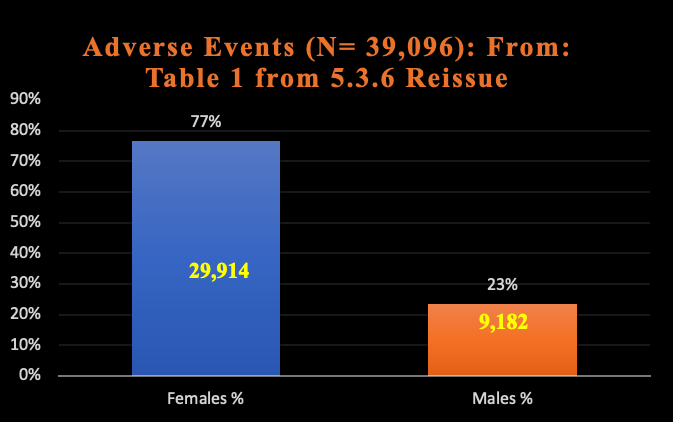
The chance this difference in reporting of adverse events between women and men is random is less than 0.001%.
The same findings apply to Adverse Events of Special Significance as shown in Chart 6 below.
Chart 6: AESI Sex Differences
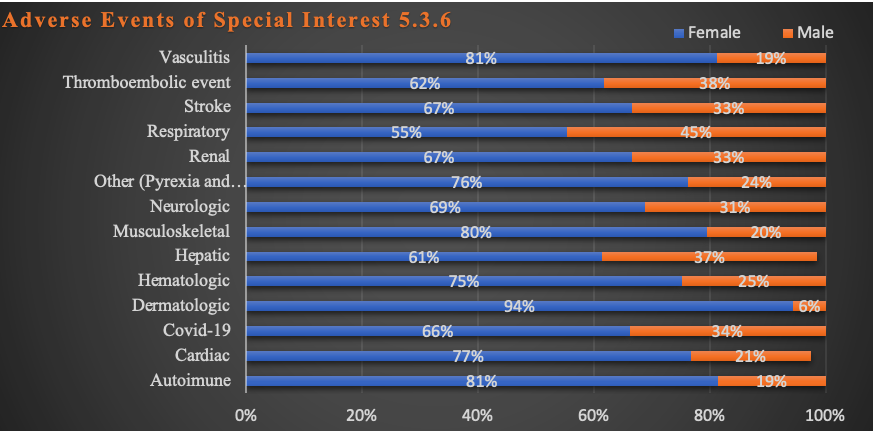
These differences are also statistically significant at p < 0.05 in all but the following: Dermatologic, Hematologic, Renal, Vascular and “Other” categories by organ system.
A subsequent report confirmed the statistically significant differences in Reproductive System and Function AEs with strong predominance of harms to women’s reproductive systems and functions compared with those of men. [https://dailyclout.io/women-have-three-times-the-risk-of-adverse-events-than-men-risk-to-the-reproductive-organs-is-even-greater-report/]
This data was collected during the same time interval as that covered by Shimabukuro, et. al. and should have been known to the CDC and FDA doctors and scientists. This information was vital to provide proper informed consent to pregnant women specifically but applies to all women.
VI. Dr. Rubin, the NEJM, FDA and CDC
“But we’re never gonna learn about how safe this vaccine is until we start giving it, that’s just the way it goes. That’s how we found out about-complications of other vaccines…And I do think that we should vote to approve it.” said FDA Vaccines and Related Biological Products Advisory Committee (VRBPAC) panel member Dr. Eric Rubin, MD, at a hearing on October 26, 2021, during an all-day session to consider use of BNT162b2 in children aged 5-11. [https://twitter.com/Techno_Fog/status/1453095851824459776 and https://townhall.com/tipsheet/scottmorefield/2021/10/26/fda-panel-member-were-never-gonna-learn-about-how-safe-the-vaccine-is-until-we-start-giving-it-n2598]
It has been debated as to whether this remark was taken out of context or not, but, either way, it remains a remarkable statement in the whole context of widespread use of novel gene therapy products and is applicable to the subject of this paper.
Dr. Rubin is Editor-in-Chief of the New England Journal of Medicine (NEJM), a once prestigious medical journal, and Adjunct Professor of Immunology and Infectious Diseases at Harvard’s T.H. Chan School of Public Health. Dr. Rubin is also a member of the FDA’s VRBPAC.
“When politics and science meet, politics wins.”. (Source unknown). [https://www.psychologytoday.com/us/blog/darwins-subterranean-world/202105/politics-and-science-losing-combination]
During 2020, the NEJM published an article unrelated to the present work that used an obviously fraudulent data set that, because of complaints from the medical community, had to be retracted. In the context of this controversy Dr. Rubin wrote the following:
Recently, substantive concerns have been raised about the quality of the information in that database. We have asked the authors to provide evidence that the data are reliable. In the interim and for the benefit of our readers, we are publishing this Expression of Concern about the reliability of their conclusions.
[See https://www.nejm.org/doi/full/10.1056/NEJMoa2007621 for an article published then retracted after numerous complaints about an obviously bogus data set was used. See for expression of concern: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7274164/pdf/NEJMc2021225.pdf. See retraction: https://www.nejm.org/doi/pdf/10.1056/NEJMe2020822?articleTools=true.]
It seems there was a precedent for faulty data sets in work published by the New England Journal of Medicine.
VII. Summary
The subject of this article was the safety determination by the CDC and FDA of LNP/mRNA experimental gene products in pregnant women.
The article fell short of any reasonable expectation of providing useful information concerning the risks to pregnant women and their babies. Accurate and reliable scientific data were not collected.
Shortcomings of the Shimabukuro, et. al. report and the body of work it reports on are abundant.
Here are 10 of them:
- The Pre-Clinical evaluation of the effects of LNP/mRNA on pregnancy was inadequate.
- Phase 1-3 Clinical Trials by Pfizer specifically excluded evaluation in pregnant women.
- The control group from the Pfizer Phase 3 trial was compromised, ending perhaps the most direct and powerful tool to understand the long-term effects of these drugs well before the required two years had elapsed.
- The Pfizer registry summarizing the first two and a half months of widespread use of LNP/mRNA identified the statistically significant warning signal of increased adverse events and adverse events of special interest after LNP/mRNA therapy in women, and this warning signal was not publicized.
- The rates of spontaneous abortion, congenital anomalies, prematurity, and neonatal death were not determined with any degree of certainty.
- 97% of the 35,691 pregnant women in the V-safe database and their babies who were injected with the experimental gene therapy drug had no outcomes recorded.
- Candidates for LNP/mRNA products were not informed of AEs, AESIs, and dose-related harms associated with these products.
- Absence of data from valid and reliable randomized controlled studies of pregnant women and their babies following treatment with LNP/mRNA products undermines a recommendation for these products in pregnant women.
- Registry data is not appropriate for analysis of never-before-used gene therapy products.
- The scientific integrity of this work was further compromised by multiple retrospective revisions of this work as revealed in the online publications June 17, 2021, September 8, 2021, and October 14, 2021.
[https://www.nejm.org/doi/full/10.1056/NEJMx210016, https://www.nejm.org/doi/full/10.1056/NEJMc2113891, https://www.nejm.org/doi/full/10.1056/NEJMc2113516, https://www.nejm.org/doi/full/10.1056/NEJMx210017j, and https://www.nejm.org/doi/full/10.1056/NEJMe2107070.]
Conclusion:
An IMMEDIATE cessation of the use of mRNA/LNP vaccines in pregnant women is mandatory until further research proves beyond doubt that they are safe to give to pregnant women.
It is necessary now to submit Freedom of Information Act (FOIA) requests for the notes of the peer reviewers, the editorial staff of the New England Journal of Medicine, and the FDA and CDC officials who raised no alarms when they saw that the vast majority of pregnant women in the CDC’s ‘V-Safe’ study – one that was invoked extensively as justification to inject millions of pregnant women with mRNA injections – were simply lost.




I am paid 114 dollars per hour to perform certain internet services online. I had no idea it was feasible, but my closest friend joined and earned $27 thousand in just five weeks by doing sac-12 this simple task. Visit for greater information about visiting this page. Anyone may obtain this right away and begin making money online.
by following the directions on this website………………. https://smartpay21.pages.dev