DailyClout Testimony Regarding Findings from Pfizer’s mRNA COVID-19 Vaccine Clinical Trial Shared at the International COVID Summit 6 (ICS6) in Tokyo, Japan

Dr. Chris Flowers, MD, represented the WarRoom/DailyClout Pfizer and Moderna Documents Analysis Project volunteers at the International COVID Summit 6 (ICS6) conference in late September in Tokyo as well as in Japan’s House of Representatives. His focus was on the hidden deaths during the clinical trial and clinical trial deletions, which reveal the fraudulent nature of the trial that allowed Pfizer’s BNT162b2 mRNA COVID-19 vaccine to be granted an emergency use authorization (EUA) by the Food and Drug Administration (FDA) in December 2020.

Dr. Chris Flowers speaking at the International COVID Summit 6 in Tokyo.
Below are Dr. Flowers’ slides and testimony script. Note that the exact phrases had to be submitted in advance to allow prior translation, so the language is scripted.

“My name is Dr. Chris Flowers. I am the medical lead of the WarRoom/DailyClout 3,400 volunteers of crowd-sourced citizens reviewing the Pfizer documents under the leadership of Dr. Naomi Wolf. Our reports have been published in two books, and the subject of today’s talk includes the peer-reviewed publication by Team 3 about our forensic examination of the Pfizer clinical trial.”
SLIDE 1: The Pfizer Clinical Trials
- The WarRoom/DailyClout Project volunteers are a mixture of medical professionals, from academia, primary care, research backgrounds, nurses, clinical trials specialists, actuaries, and many others.
- We are all unpaid volunteers with no conflicts of interest.
- We have reviewed every page of the documents released by the FDA following a Freedom of Information Act request and a court order.

SLIDE 2: The Pfizer Clinical Trial C4591001
- Our question is, “Can we trust the clinical trials used to approve these modified mRNA vaccines?”
- The committee, VRBPAC (Vaccines and Related Biological Products Advisory Committee), recommending this new preventative treatment met on December 10, 2020, and the FDA formally approved Pfizer’s COVID vaccine the next day.
- A paper about the trial was published the day after approval in the New England Journal of Medicine.
- There were nearly 44,000 subjects in the trial divided into two groups – placebo recipients and intervention recipients.
- The groups were balanced for age, gender, and demographics – BUT there were MORE deaths in the vaccinated group.
- We reviewed all the individual patient case report forms (CRFs) and medical notes of the six months of data when they were released by the FDA
- The data at six months showed 38 deaths (21 vaccinated, 17 placebo).
- At the approval meeting on December 10, 2020, Pfizer reported six deaths (two vaccinated, four placebo).
- The CRFs show at least eight deaths at that time (four vaccinated, four placebo).
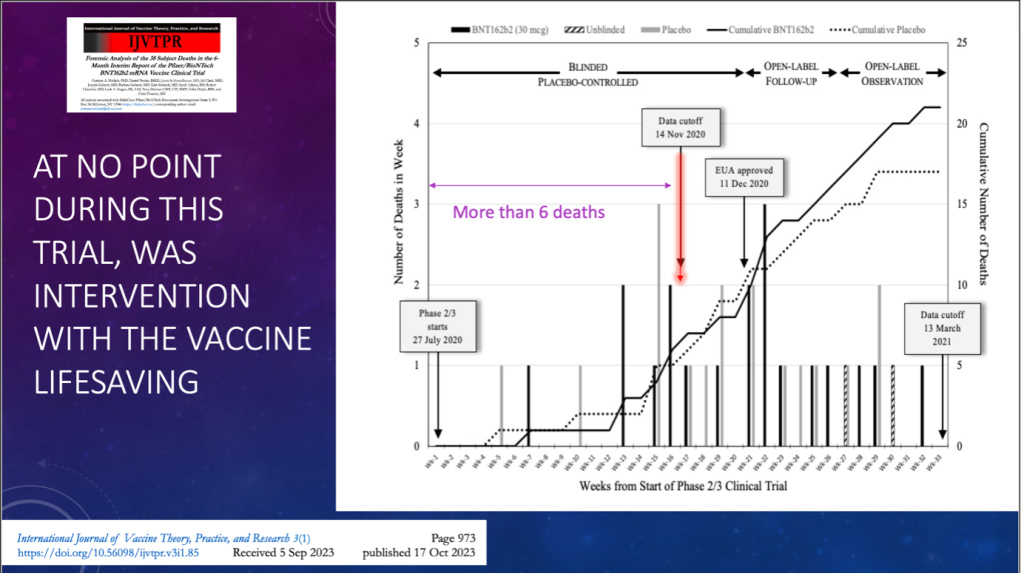
SLIDE 3: Peer-reviewed published paper on the analysis of the clinical trial deaths
- The graph shows the number of deaths over time by week.
- The graph also shows the timings of the ‘data cut-off point’ and the date of approval of the emergency use authorization (EUA).
- This shows no difference in number of deaths between those getting Pfizer’s BNT162b2 vaccine or the placebo.
- After EUA approval, almost all the placebo recipients from the trial received the vaccine, and the deaths among those receiving the vaccine continued to climb.
- At no point in the trial was there evidence that this intervention would save people’s lives.
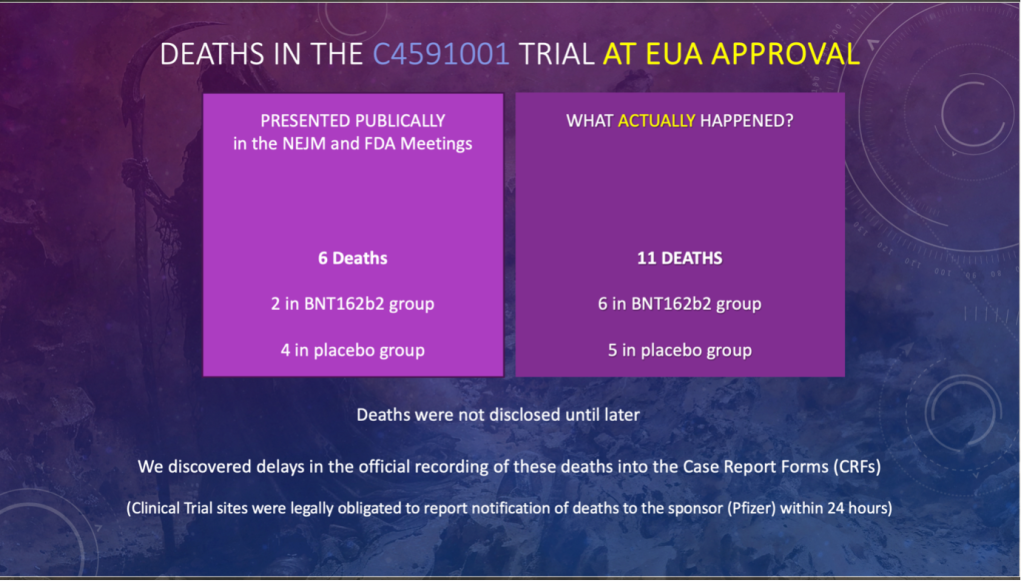
SLIDE 4: Deaths in the C4591001 TRIAL at EUA Approval
- Here is a visual presentation of the same data with ALL the data not available at the time of approval.
- The reporting of deaths due to the intervention was delayed.
- The deaths were NOT disclosed until later.
- We found these delays in reporting by reviewing the CRFs and were able to show evidence of two deaths that Pfizer knew about but did not disclose at the time of the data submission for the EUA.
- Clinical trial sites were legally obligated to report notifications of death to the sponsor (Pfizer) within 24 hours.
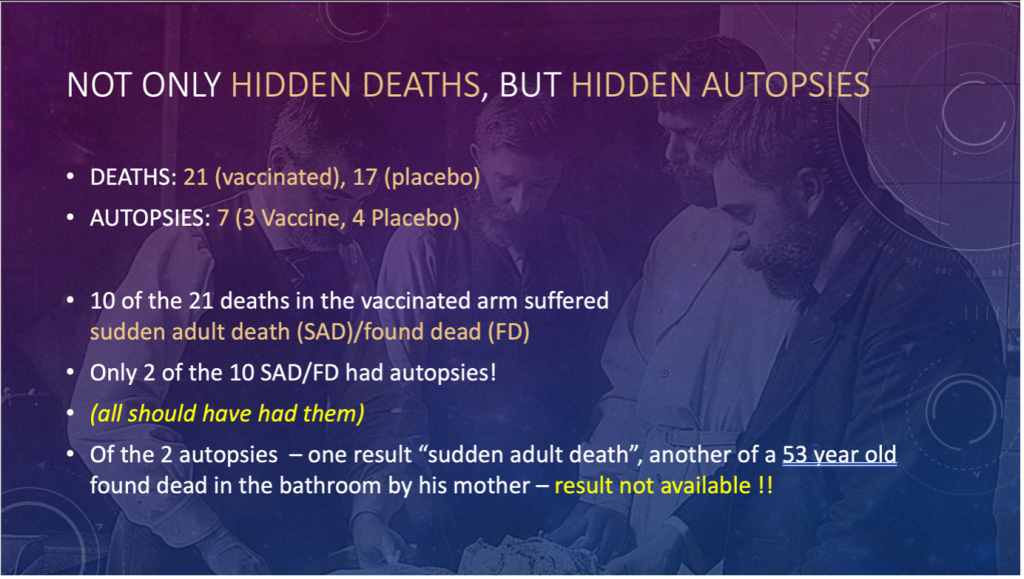
SLIDE 5: Not Only Hidden Deaths, But Hidden Autopsies
- Out of the 38 total patient deaths in the trial, only seven autopsies were performed (18%).
- Out of the 21 deaths in the vaccine arm of the trial, 10 suffered SUDDEN ADULT DEATH (SAD) or were Found Dead (FD).
- Only two of these had autopsies. (EVERYONE SHOULD HAVE AN AUTOPSY IN THAT CIRCUMSTANCE.)
- Of those, one had a diagnosis of Sudden Adult Death – unknown.
- The other was a 53-year-old man found dead in the bathroom by his mother – the CRFs state, “RESULT NOT AVAILABLE”! And they are still not publicly available three years later.
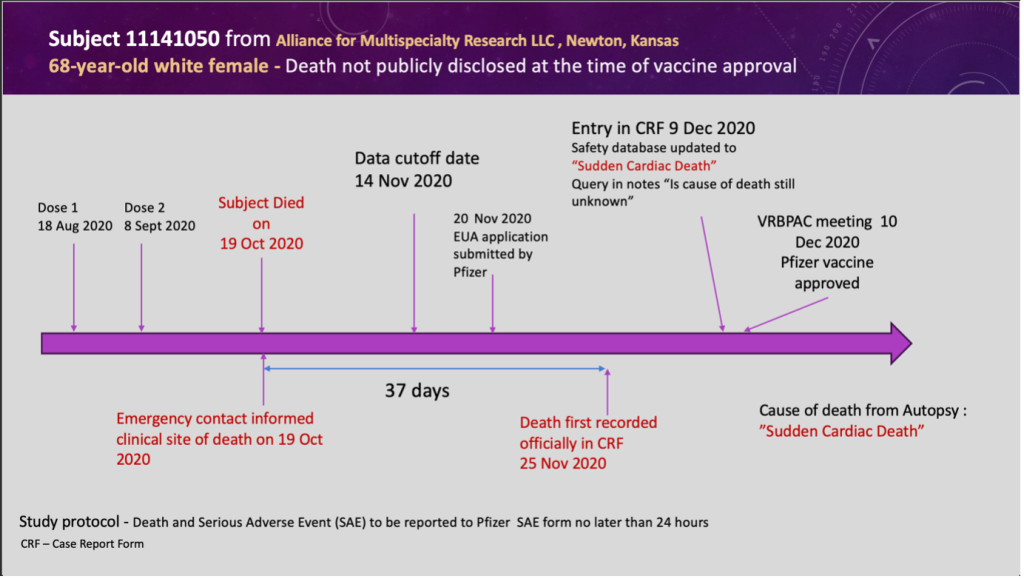
SLIDE 6: An Example of Delayed Reporting of Autopsy Results
- 68-year-old female – death NOT publicly disclosed at the time of drug approval.
- The subject died on October 19, 2020, and the site was notified the same day by the patient’s emergency contact.
- The death was not officially recorded in the CRF until November 25, 2020, a delay of 37 days.
- The autopsy result was Sudden Adult Death – cause unknown.

SLIDE 7: Data Cut-Off Point and Delayed Recording of Deaths
- There were, in fact, 11 clinical trial deaths at the time of the data cut-off on November 14, 2020.
- Only eight of those had been recorded, and only six were disclosed by Pfizer and reported in the New England Journal of Medicine by Polack et al.
- The range of delays was from one day to 37 days.
- They recorded 80% of the placebo group deaths and only 33% of the deaths in the vaccinated group.

SLIDE 8: Other Issues with the Trial
- DESTRUCTION OF THE PLACEBO GROUP
- Placebos are important because they provide a baseline when assessing the long-term safety of a drug.
- After the EUA was granted, the placebo group was offered the vaccine, and most placebo participants took it.
- By removing the control (i.e., placebo) group, the baseline was removed.
- Similarly, in the Pfizer pediatric COVID vaccine trials, the subjects were unblinded and received a third dose, which destroyed the ability to track long-term safety in the children’s trial.

SLIDE 9: Other Issues with the Trial
- Women who became pregnant during the Pfizer clinical trial were dropped from the trial without follow-up.
- Issues with subject randomization (especially in selected populations – e.g., Argentina).
- Large numbers of subjects logged as ‘Discontinued Subjects’ with no follow-up
- What happened to 2,100 unblinded placebo subjects?
- Missing CRFs: Is Pfizer hiding them, or did they not do the required follow-up?
- Information still withheld by Pfizer and why this is critical: autopsies, pregnancy test results, blood cell counts, troponin levels, and more.
- Obfuscation of diagnosis: preferred terms, diagnosis inaccuracy, diagnosis alteration/manipulation
- The BNT162b2 vaccine granted EUA was a different product from what the vast majority of clinical trial participants received. It also contained unacceptable levels of undisclosed contaminants.
- And many more

SLIDE 10: CONCLUSIONS
- Pfizer’s COVID-19 vaccine did NOT prevent death.
- It was not tested for long-term safety.
- The effects on transmission were never studied.
- More trial subjects died in the BNT162b2 group than the placebo group.
- Smaller than expected number of autopsies in patients who ‘died suddenly.’
- Huge numbers of trial subjects were lost to follow-up.
- The clinical trial used to justify the public use of this product was flawed and misreported.
- Who will be held to account?
The day after my presentation, we proceeded to give the testimony in Japan’s House of Representatives, where a senator and a representative were present, and the session was recorded for use in their parliamentary inquiries.
Here we are leaving the hotel for Parliament.

Left to right: John Kage, Jason Christoff, William Makis, James Lindsay, Chris Flowers.

Dr. Chris Flowers representing the WarRoom/DailyClout Pfizer and Moderna Documents Analysis Project at the International COVID (Crisis) Summit 6 in Tokyo, Japan.
Order The Pfizer Papers: Pfizer’s Crimes Against Humanity.





