What Does Disappearing Immunity To Covid-19 Mean For A Vaccine?
The first results of Moderna’s Covid-19 vaccine trials were officially published Tuesday and the news was good—patients injected with the mRNA-based vaccine produced antibodies that fight the Covid-19 virus.
Just how good is this good news? That depends on whether or not you accept the fundamental assumption underlying this generation of Covid-19 vaccines as fact. To proclaim these trials a success is to take for granted that exposing people to viral proteins will trigger a vigorous, long-lasting immune response. But studies of the molecular biology of SARS-CoV-2, along with the natural history of the coronavirus family, may offer evidence to the contrary.
Vaccines don’t act as shields, protecting you from a disease. Instead they act more as alarms. Just like a fire alarm won’t put out the fire, a vaccine won’t prevent you from infection. They’re just a means to alert your immune system early on that a danger exists. In the end, it’s your immune system—including those antibodies—that does all the work.
Several recent studies have found that antibodies to SARS-CoV-2 decline in strength and number in the weeks and months following infection. One study describes a decline in even neutralizing antibodies, which are thought to be protective against reinfection and disease. While this decline is not without precedent and is in fact typical of human coronavirus infections, the question is what it portends for the pandemic and for the success of Covid-19 vaccines.
“Get it and forget it”
SARS-CoV-2 is broadly similar to the four coronaviruses that cause about one third of all common colds. Each year, the same four viruses infect us the world over, sweeping the Northern Hemisphere from December to February; south of the Equator from May to July; and in the tropics, year-round. These waves of infection, which with rare exceptions cause minor symptoms only, have repeated year in and year out since the discovery of the virus in the 1960s. The ability of this coronavirus quartet to persist absent alteration is highly unusual. Influenza infections occur annually, too, but the dominant strains differ each time to evade the population’s protective and persistent immune responses.
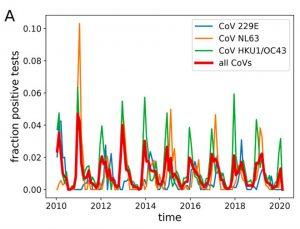
Source: POTENTIAL IMPACT OF SEASONAL FORCING ON A SARS-COV-2 PANDEMIC DOI: HTTPS://DOI.ORG/10.4414/SMW.2020.20224 PUBLICATION DATE: 16.03.2020
In the 1970s two independent teams of medical researchers conducted experiments to determine whether or not the same coronavirus strain might reinfect and give a cold to the same person. Volunteers who were deliberately exposed to the virus contracted colds and recovered. A year later, they were again exposed to the same virus—and again were infected and developed cold symptoms. These experiments established that protective immunity to the cold-causing coronavirus is short-lived.
I call this phenomenon “get it and forget it,” and it describes the interaction between these viruses and our immune systems that is so unique. Confronted with a cold-causing coronavirus, our bodies evidently forget that we were infected at all. For us, this leaves us susceptible to annual colds, which are generally harmless but a nuisance besides. For the viruses, this is a winning strategy, as it rids them of the need to change to survive. At present, we don’t understand coronaviruses in sufficient detail to know why our immunity to them so short-lived.* What we do know is that if SARS-CoV-2 behaves as its coronavirus cousins do, Covid-19 is sure to become a seasonally recurring pandemic.
A vanishing act
As I mentioned previously, it has already been confirmed that immunity to SARS-CoV-2 fades quickly after infection. When viral infection occurs, a type of antibody called IgM appears within one to two weeks. IgM antibodies mobilize against the virus, then begin to disappear in the weeks and months following infection. Two to three weeks after an infection has cleared, IgG antibodies appear.
It is the case for many viruses, such as those that cause most childhood diseases, that reasonably high levels of IgG antibodies persist for many years. It is not the case for SARS-CoV-2. Studies show that the IgG antibodies we produce while recovering from Covid-19 decline rapidly. Not only that, but in blood samples from some convalescent Covid-19 patients, IgG antibodies and even IgM antibodies are altogether absent. (That these patients were, in fact, infected was confirmed by successive PCR tests.)
How does SARS-CoV-2 engineer this disappearing trick, and what implications would the mechanism have for the development of vaccines? One possibility is that the coronavirus proteins have evolved to become weak immunostimulants. If this is so, it will be a problem for Covid-19 vaccine candidates that use natural variants of the virus proteins as antigens. To be successful, their makers will have to engineer proteins that differ from those in the virus itself—not an impossible task, but a difficult one that would take time. We won’t know the answer until we learn how well the vaccines protect against infection, and how long protection lasts.
Manipulating immune memory
Another possibility is that coronaviruses have developed the means to manipulate the human immune response to its own advance. Coronaviruses have carved out an interesting ecological niche for themselves. When a coronavirus enters the body, it down-modulates the immune system, reducing our immune response. In doing so, the virus gains a toehold allowing it to spread. Even if the immune system is able to eventually clear the virus, the damage has been done. By down-modulating our initial immune reaction, the coronavirus has ensured that our long-term response to the virus isn’t as powerful as it ought to be. Immunity fades and our bodies essentially forget that we were ever infected.
In another, the virus could actively compromise the memory response upon re-infection. Remember that vaccines, once injected, don’t shield the body from infection. Instead, they equip the immune system with a sophisticated alarm system—one that will trigger a vigorous, rapid immune response whenever the invading virus sets off the alarm. Immune memory cells, trained by the vaccine to mobilize at the sound of the alarm, allow the body to mount a full-blown immune response in days rather than weeks. If SARS-CoV-2 does have the ability to impede immune memory, that means it can effectively disconnect the alarm. A virus with the potential to compromise the memory response would pose a difficult vaccine challenge indeed.
There are hints that coronaviruses, SARS-CoV-2 included, may employ several immune evasion techniques. We already know that SARS-CoV-2 does produce one or more proteins that actively interfere with immune function. The orf-3b gene product specifically interferes with precisely those signals impotent for initiating the production of anti-viral antibodies and T-cells. The orf3b protein down-regulates the production of interferon-gamma and IL-2, both necessary for initiating the production of anti-viral antibodies and T-cells.
The ability of orf3b to interfere with immune activation is very likely the tip of a very large iceberg when it comes to altering the immune response. At least 9 SARS virus proteins and 6 MERS virus proteins are known to interfere with the host immune response. It is very likely that SARS-CoV-2 also carries a full complement of similar genes that collectively may help explain three usual features of infection, antibody-negative convalescents, rapidly fading antibody levels, and re-infection.
Immune reactions to SARS-CoV-2 infection and disease are complex, and we knew from the beginning that developing a vaccine that outwits the virus wouldn’t be easy. We would be very lucky to get it right on the first try. If we’re not blessed with such good fortune, we must buckle down and recognize that we’re in this for the long haul—and throw our support into the prolonged research effort necessary to ensure success.
* One reason we don’t have the information we need is the start-stop nature of coronavirus research funding. For 40 years post-discovery, coronavirus research was a rare specialty. Research on many other viruses took precedence—mostly those that kill, such as HIV/AIDS, polio, smallpox, Ebola, and respiratory syncytial virus. It was not until the advent of SARS in 2003, followed by MERS, that research on these viruses was well-funded and scientists had the opportunity to begin to understand them in more detail. As SARS waned and the potential for MERS to cause pandemic disease disappeared, much of the funding for coronavirus vanished. We’re just now playing catch-up to understand some of the details of the interaction of SARS-CoV-2 with the human body at the level we understand HIV after more than 35 years of intense research. We’re filling in the gaps, but we have a long way to go.
Reprinted with permission from Forbes.




