Report 43: Twenty-Two Cases of Rare Myocarditis by February 2021, Yet Pfizer Said No “New Safety Issues.” FDA Waits Until June 25, 2021, to Include Myocarditis Risk in Fact Sheets.


As initially reported by Chris Flowers, M.D., on DailyClout.io in April 2022, myocarditis – inflammation of the heart muscle (a.k.a., myocardium) that can reduce the heart’s ability to pump blood as well as cause chest pain, shortness of breath, and rapid or irregular heart rhythms (a.k.a., arrhythmias) [https://www.mayoclinic.org/diseases-conditions/myocarditis/symptoms-causes/syc-20352539] – is a serious adverse event (SAE) that the Food and Drug Administration (FDA) knew about in May 2021 when it renewed the Emergency Use Authorization (EUA) for Pfizer’s mRNA COVID-19 vaccine, BNT162b2. [https://dailyclout.io/pfizer-vaccine-fda-fails-to-mention-risk-of-heart-damage-in-teens/] This report brings to light additional information on myocarditis from Pfizer’s ”5.3.6 Cumulative Analysis of Post-Authorization Adverse Event Reports of PF-07302048 (BNT162b2) Received Through 28-Feb-2021.” [https://www.phmpt.org/wp-content/uploads/2022/04/reissue_5.3.6-postmarketing-experience.pdf]
As early as February 2021, Pfizer had 22 cases of myocarditis, less than three months into the mRNA COVID-19 mass vaccination program in the United States. In fact, the FDA did have this information when 5.3.6. was given to them by Pfizer on April 30, 2021.
These cases had onset within seven days, with a median onset of two days, and Pfizer concluded that there were no “new safety issues.” [https://www.phmpt.org/wp-content/uploads/2022/04/reissue_5.3.6-postmarketing-experience.pdf and https://my.clevelandclinic.org/health/diseases/22129-myocarditis] Speaking as a physician, this early post-authorization data, in and of itself, warns of the “increased risks of myocarditis,” “particularly within 7 days,” as today is warned in the COMIRNATY® package insert. [https://labeling.pfizer.com/ShowLabeling.aspx?id=15623&format=pdf]
Reviewing the reissue of Pfizer’s “5.3.6 Cumulative Analysis of Post-Authorization Adverse Event Reports of PF-07302048 (BNT162b2) Received Through 28-Feb-2021” (received by the FDA on April 30, 2021 and then published by the FDA on April 1, 2022, with original FDA publication on November 17, 2021), this physician was struck by the high number of rare adverse events (AEs) in Table 7 – e.g., myocarditis:
- In fewer than three months of post-authorization reporting (mid-December 2020 through February 28, 2021).
- With an Adverse Event of Special Interest (AESI) category median relevant event onset latency of less than 24 hours.
- With a conclusion of no new safety issues [https://www.phmpt.org/wp-content/uploads/2022/04/reissue_5.3.6-postmarketing-experience.pdf].
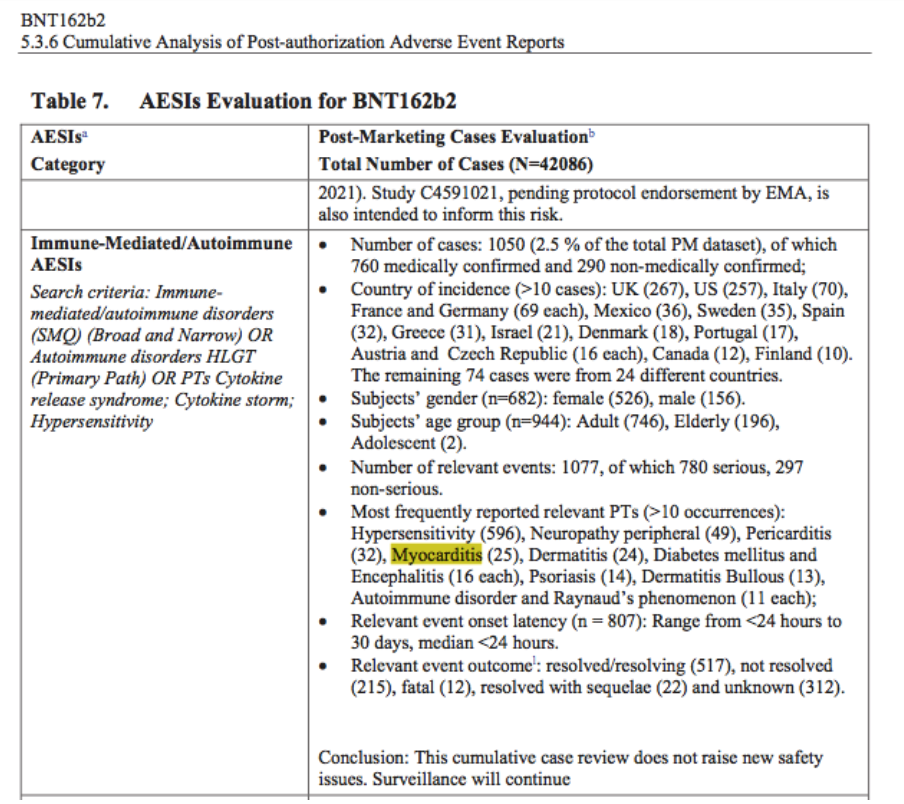
Figure 1: From p. 20 of “5.3.6 Cumulative Analysis of Postmarketing Adverse Event Reports”
Without seeing the Individual Case Safety Reports (ICSRs) that made up the pooled data in Table 7, could anyone, outside of Pfizer, suspect a new safety issue on his or her own?
Pfizer had to report all post-authorization SAEs to the Vaccine Adverse Reporting System (VAERS), so now the public can see actual, vetted ICSR data held, as of February 28, 2021, and come up with their own conclusion.

Based on a VAERS query, one can view the SAEs received by Pfizer:
- Through February 28, 2021
- For the Preferred Term (PT) myocarditis (Symptoms)

Figure 2: Screenshot of VAERS search criteria used on 7/15/2022.
[https://wonder.cdc.gov/vaers.html]
This shows that 22 cases of myocarditis were received by Pfizer (have an Mfr/Imm Project Number) through February 28, 2021, and assessed by its medical review team prior to submitting to VAERS:
- In fewer than three months of post-authorization reporting, Pfizer had twenty-two (22) reports of a rare condition, PT: myocarditis.
- Removing the six Pfizer reports with an unknown onset interval, the median (0000011222334557) event onset latency was two days – i.e., two days post-vaccination.
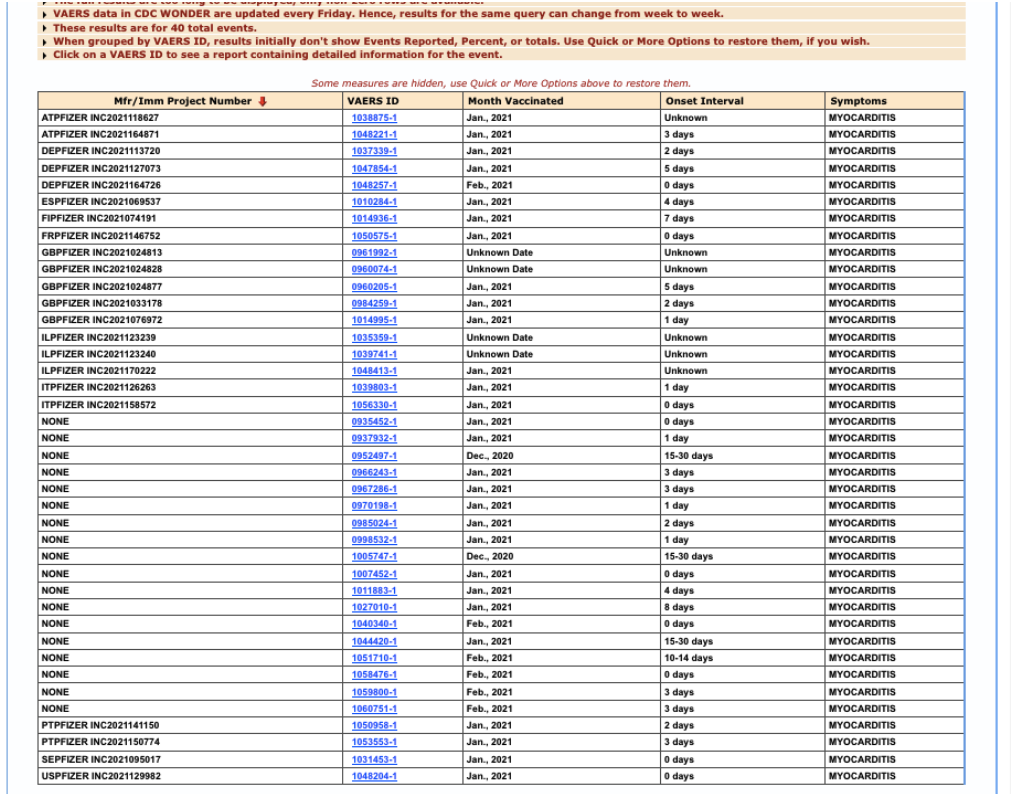
Figure 3: VAERS reports of myocarditis, received by Pfizer, through 2/28/2021.
.
Today, COMIRNATY® carries a warning regarding myocarditis and pericarditis.

Figure 4: COMIRNATY’s label warning about myocarditis and pericarditis.
[https://labeling.pfizer.com/ShowLabeling.aspx?id=15623&format=pdf]
Editing the VAERS query from Month Vaccinated to Vaccine Dose, and setting aside all unknowns, shows that four myocarditis cases occurred within seven days following the first dose and eight cases within seven days following the second dose.
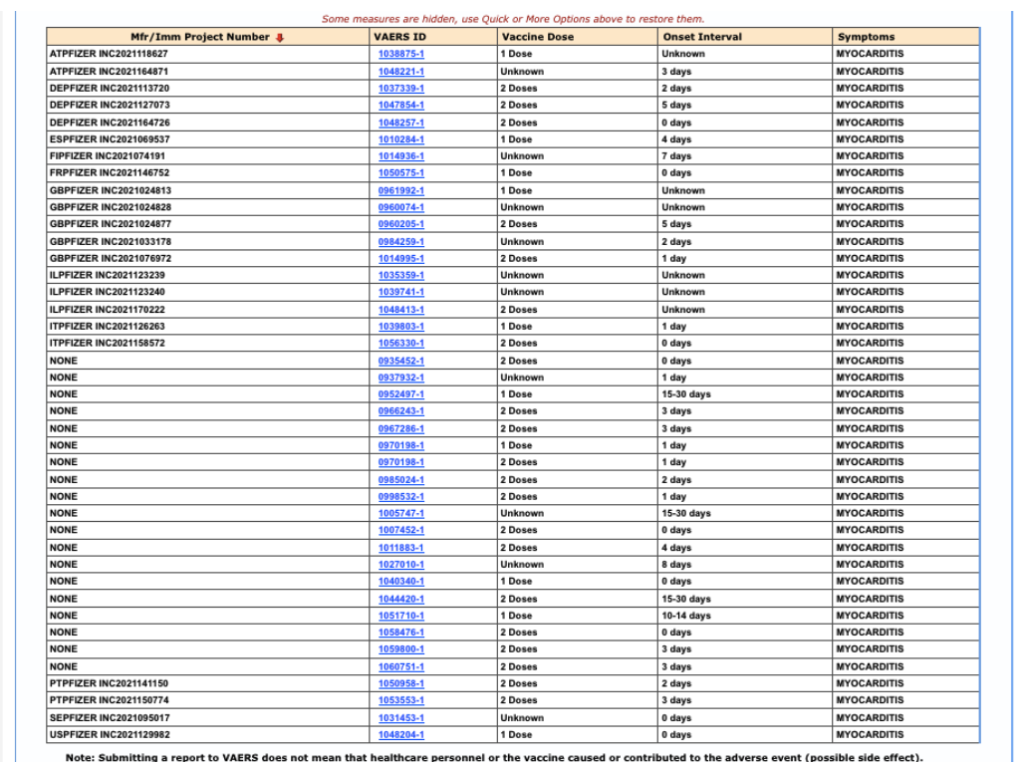
Figure 5: VAERS query of myocarditis based on vaccine dose.
And, in people under 40 years of age, one finds there were five cases received by Pfizer, at least three with onset within seven days following the second dose:
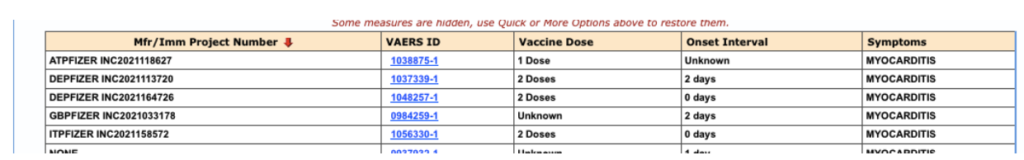
Figure 6: VAERS reports of myocarditis, received by Pfizer, in people under 40 through 2/28/2021.
Lastly, two of the Pfizer reports have a written causality assessment by the medical reviewer. Even though all spontaneous reports have implied causality for regulatory reporting purposes, meaning the adverse event (AE) is suspected to be due to the suspect drug or biological product, many companies provide the medical reviewer’s assessment of causality in the report narrative. [https://www.fda.gov/media/73593/download]
Below are details on the two reports, each acknowledging the temporal (i.e., time) relationship between the myocarditis event and the vaccine being given:
| Mfr/Imm Project Number | VAERS ID | Date Vaccinated | Date of Onset | Patient Age/Sex | Dose Number | Excerpt from
Medical reviewer assessment |
| FRPFIZER INC2021146752 | 1050575-1 | 2021-01-14 | 2021-01-14 | 53/male | 1 | Based on the information currently available, a possible contributory role of the suspect drug in the reported event Myocarditis cannot be completely excluded given the known suspect drug profile and/or implied temporal association. |
| GBPFIZER INC2021024877 | 0960205-1 | 2021-01-04 | 2021-01-09 | 56/female | 2 | Based on the current available information and the plausible drug-event temporal association, a possible contributory role of the suspect product BNT162B2 to the development of event Myopericarditis cannot be totally excluded. |
How can anyone who has reviewed the 22 myocarditis ICSRs, with all known onsets within seven days post-vaccination, agree with Pfizer’s published conclusion, received by the FDA on April 30, 2021, of no new safety issues?
Prior to the FDA’s initial myocarditis warning on June 25, 2021, Pfizer had received (at least through May 2021), 288 additional reports of myocarditis particularly within seven days. [https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-june-25-2021]
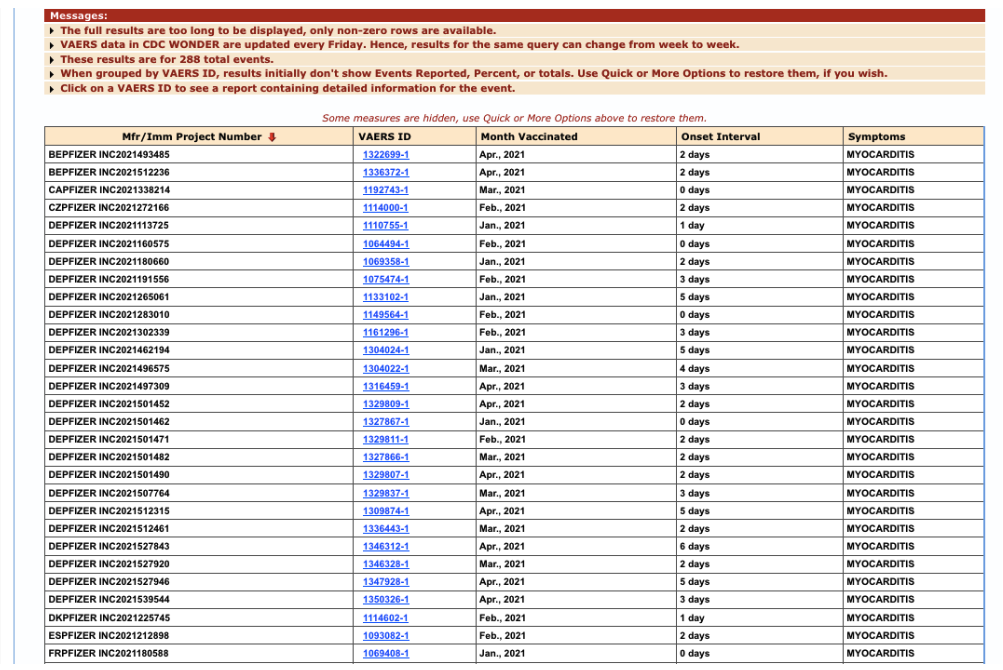
Figure 7: Additional VAERS reports of myocarditis through May 2021, particularly within seven days.
A new safety issue for myocarditis was apparent at the time of the completed “5.3.6 Cumulative Analysis of Post-authorization Adverse Event Reports,” received by the FDA from Pfizer on April 30, 2021. [https://www.phmpt.org/wp-content/uploads/2022/04/reissue_5.3.6-postmarketing-experience.pdf] Identification and communication of this concern at the time should have served as the initial adverse event warning announcement. Instead, the FDA waited until June 25, 2021, to issue a formal announcement – a two-month delay.




“Twenty-Two Cases of Rare Myocarditis by February 2021, Yet Pfizer Said No ‘New Safety Issues.’ FDA Waits Until June 25, 2021, to Include Myocarditis Risk in Fact Sheets.”
As Gomer Pyle would say, “Surpriiiiise, surpriiiiiise!!!!” 😛
And speaking of vaccine companies, apparently Moderna is sponsoring the U.S. Open tennis tournament, which is systematically excluding athletes brave enough to refuse the jab, like Novak Djokovic, the top male tennis player in the world. Well, absolutely NO conflict of interest there!!! 😛 True, the so-called U.S. government is refusing entry to most unjabbed foreigners like him, but the U.S. Open could’ve still applied for a special exemption for him, which they most decidedly did *not*. Anyway, Daily Clout might want to consider posting an article about this farce.
Don’t know if you saw this on David Martins’ show last week: https://youtu.be/aS2DFVbPuqo
It looked fairly interesting to me…