Report 41: 2021 CDC and FDA Misinformation – Retroactive Editing, Erroneous Spontaneous Abortion Rate Calculation, Obfuscation in the New England Journal of Medicine

The following article is a follow-up to Dr. Chandler’s report, “Data Do Not Support Safety of mRNA COVID Vaccination for Pregnant Women,” which reviewed “Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons,” New England Journal of Medicine, April 21, 2021, and June 17, 2021. To best understand this report, please read the previous report first.
Fortitude is required for anyone who endeavors to try to understand the surveillance and reporting of the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) concerning safety of Pfizer and Moderna’s LNP/mRNA gene therapy products in pregnant women in the year following widespread distribution of these products (December 14, 2020, until December 14, 2021) as reported in the New England Journal of Medicine (NEJM), Research Square, Obstetrical and Gynecological Survey and Obstetric Anesthesia Digest during calendar year 2021.
[Shimabukuro, et al, NEJM, April 21, 2021/October 14, 2021; DOI: 10.1056/NEJMoa2104983, https://www.nejm.org/doi/full/10.1056/NEJMoa2104983. https://www.nejm.org/doi/full/10.1056/NEJMe2107070. https://www.nejm.org/doi/full/10.1056/NEJMc2113516. https://www.researchsquare.com/article/rs-798175/v1. https://journals.lww.com/obgynsurvey/Abstract/2021/12000/Preliminary_Findings_of_mRNA_COVID_19_Vaccine.7.aspx. https://journals.lww.com/obstetricanesthesia/Abstract/2021/12000/Preliminary_Findings_of_mRNA_Covid_19_Vaccine.2.aspx.]
The CDC used two voluntary registries to track pregnant women after they were injected with at least one dose of LNP/mRNA genetic therapy drugs, the V-safe/Pregnancy Registry that was created for Covid-19 specifically and the long-standing Vaccine Adverse Event Reporting System (VAERS) that has tracked adverse events following administration of vaccines since 1990.
[https://www.cdc.gov/vaccinesafety/pdf/vsafe-pregnancy-surveillance-protocol-508.pdf.
https://www.cdc.gov/vaccinesafety/pdf/V-safe-Protocol-508.pdf.
Advice to self: Be prepared to download and save documents before they are changed online. Make liberal use of screenshots for important discoveries, as information in the digital age can be very fluid, unlike the memory hole of Orwell which required mechanical incineration of unfavorable information in hard copy form and continuous issuance of updated versions of the past. We now have a digital version of the memory hole.
Registry Data
The protocol for V-safe is currently in a 69-page Version 4 from March 10, 2022, entitled “V-safe Active Surveillance for Covid-19 Vaccine Safety and Amendment.” [https://www.cdc.gov/vaccinesafety/pdf/vsafe-pregnancy-surveillance-protocol-508.pdf and https://www.cdc.gov/vaccinesafety/pdf/V-safe-Protocol-508.pdf] Perhaps you will be able to find Version 1, but it will be more productive to move on to the first published results from these databases by Shimabukuro, et al. in the April 21, 2021, issue of NEJM. [Shimabukuro, et al, NEJM, April 21, 2021/October 14, 2021; DOI: 10.1056/NEJMoa2104983, https://www.nejm.org/doi/full/10.1056/NEJMoa2104983.]
Following publication, Shimabukuro’s article had a very confusing history. A spreadsheet tracking the changes in publications by authors from the CDC and FDA reporting on data queries from the CDCs V-safe/Pregnancy Registry and VAERS 12/14/2020 through 2/28/2021 is attached as Exhibit I.
In brief, publication history of the “Preliminary Findings…” article and its progeny in the year following the late 2020 Emergency Use Authorization (EUA) is as follows:
- April 21, 2021, NEJM: Shimabukuro, et al. Original Article published. [Shimabukuro, et al, NEJM, April 21, 2021/October 14, 2021; DOI: 10.1056/NEJMoa2104983, https://www.nejm.org/doi/full/10.1056/NEJMoa2104983.]
- June 17, 2021, NEJM recycled “Original Article” from April 21, 2021, published. [Shimabukuro, et al, NEJM, April 21, 2021/October 14, 2021; DOI: 10.1056/NEJMoa2104983, https://www.nejm.org/doi/full/10.1056/NEJMoa2104983.]
- Reportedly, the June 17, 2021, republished “Original Article” was modified retroactively on September 8, 2021, changing the “original” text online. [https://www.nejm.org/doi/full/10.1056/NEJMx210016]
- Zauche, et al. made a confusing second attempt to put forth a number for the rate of spontaneous abortions in the August 9, 2021, issue of Research Square. [https://www.researchsquare.com/article/rs-798175/v1] This material was republished in the October 14, 2021, NEJM with the bulk of the paper appearing in the form of a Supplement. [https://www.nejm.org/doi/full/10.1056/NEJMc2113891]
- The September 9, 2021, edits of the June 17, 2021, Shimabukuro, et al. paper were reported in authorless “Corrections” in the October 14, 2021, issue of NEJM. The June 17, 2021, online version was modified retroactively. [https://www.nejm.org/doi/full/10.1056/NEJMe2107070]
- The abstract from the edited June 17, 2021, version of the NEJM article was published in the December issue of Obstetrical and Gynecological Survey. [https://journals.lww.com/obgynsurvey/Abstract/2021/12000/Preliminary_Findings_of_mRNA_COVID_19_Vaccine.7.aspx] The study by Zauche, et al. was not mentioned. [https://www.researchsquare.com/article/rs-798175/v1]
- The full form of the September 8, 2021, edited June 17, 2021, republication of the April 21, 2021, original was republished in the December issue of Obstetrical Anesthesia Digest. [https://journals.lww.com/obstetricanesthesia/Abstract/2021/12000/Preliminary_Findings_of_mRNA_Covid_19_Vaccine.2.aspx] The analysis by Zauche, et al. was not mentioned.
- As of September 2022, the April 21, 2021, NEJM publication was no longer available online.
- As of September 2022, the September 8, 2021, NEJM corrections was no longer available online.
In Zauche, et al. CDC and FDA authors were joined by colleagues from the United States Department of Energy, United States Public Health Service, National Institute of Environmental Health Sciences, and the Department of Mathematics at the University of California – San Diego in the special analysis of Zauche, et al. [https://www.nejm.org/doi/full/10.1056/NEJMc2113891]
No Updates in 2021 as Pregnancies Complete
By December 2021, all 3,958 pregnant women entered into the V-safe Pregnancy Registry would have completed their pregnancies yet, other than Zauche, et al., no new data was added to the various reports from April through December 2021. [https://www.researchsquare.com/article/rs-798175/v1 and https://journals.lww.com/obgynsurvey/Abstract/2021/12000/Preliminary_Findings_of_mRNA_COVID_19_Vaccine.7.aspx] Even after Zauche, et al. was published in August and republished in October, the December versions of Shimabukuro, et al. report on the same data set reported in April 2021.
[Shimabukuro, et al, NEJM, April 21, 2021/October 14, 2021; DOI: 10.1056/NEJMoa2104983, https://www.nejm.org/doi/full/10.1056/NEJMoa2104983.
Shimabukuro, et al. and Edits
Shimabukuro, et al. reported “Preliminary Findings of mRNA Covid-19 Safety in Pregnant Persons,” April 21, 2021, in the New England Journal of Medicine. [Shimabukuro, et al, NEJM, April 21, 2021/October 14, 2021; DOI: 10.1056/NEJMoa2104983, https://www.nejm.org/doi/full/10.1056/NEJMoa2104983.] One may be able to find this in a library, but search online and you are likely to find only the June 17, 2021, version that was retrospectively edited on September 8, 2021, changing the June 17, 2021, version of the April 21, 2021, original. The actual September edit notification has not yet been located online, but the edit was documented later in the October 14, 2021, issue of NEJM. [https://www.nejm.org/doi/10.1056/NEJMx210016] The edits and versions of Shimabukuro, et al. are detailed in Exhibit II.
Riley and Edits
In the same June 17, 2021, issue that had the Shimabukuro, et al. republication there was an editorial by Dr. Laura Riley, MD, Chairman of the Department of Obstetrics and Gynecology at Weill Cornell Medical School. [https://www.nejm.org/doi/full/10.1056/NEJMe2107070 and https://directory.weill.cornell.edu/person/profile/lar9110] Exhibit III.
Dr. Riley is also a member of the New England Journal of Medicine Editorial Board (Exhibit III) In her editorial, Dr. Riley stated:
“… clinicians relied on developmental and reproductive animal data from Moderna that showed no safety concerns, and there was no biologically plausible reason that the mRNA technology would be harmful in pregnancy.” [https://www.nejm.org/doi/pdf/10.1056/NEJMe2107070?articleTools=true, p. 2342.]
This statement is simply not consistent with the fact that the LNP/mRNA products were not thoroughly evaluated in pre-clinical studies and received no formal testing by Pfizer in pregnant women as noted in the Polack, et al report of Phase 3 clinical trials.
[https://www.nejm.org/doi/full/10.1056/NEJMoa2034577 and https://robertchandler.substack.com/p/pfizer-pre-clinical-studies-review]
Dr. Riley went on to note that Shimabukuro, et al. reported spontaneous abortion in 12.6% of the 827 registry participants who had completed pregnancies, a figure obtained by dividing 104 spontaneous abortions in the first 20 weeks by the 827 completed pregnancies.
The problem with this calculation is that 700 of the 827 pregnancies followed to completion were given the LNP/mRNA product in their third trimester and should not have been included in the denominator. The various calculations that have been attempted with these data were presented in an earlier article. [https://dailyclout.io/data-do-not-support-safety-of-mrna-covid-vaccination-for-pregnant-women/]
This error was addressed in a stealth edit in a September 8, 2021, NEJM message that was fully reported in the October 14, 2021, issue of NEJM as noted in Exhibit III. Other edits were also made, as noted in Exhibit III. [https://www.nejm.org/doi/full/10.1056/NEJMx210017?query=recirc_curatedRelated_article]
Sun Correspondence
Dr. Hong Sun, PhD of Antwerp, Belgium questioned the calculation of 12.6% spontaneous abortion rate pointing out that the denominator included 700 pregnant women who received their first dose in the trimester and should not have been included in a calculation of spontaneous abortion rate. Exhibit IV. [https://www.nejm.org/doi/full/10.1056/NEJMc2113516]
It is not clear when Dr. Sun’s letter was received, but this note is in the October 14, 2021, publication of his “Correspondence”:
“This letter was published on September 8, 2021, at NEJM.org.” [https://www.nejm.org/doi/full/10.1056/NEJMc2113516]
However, there is a citation in Dr. Sun’s correspondence that references the Shimabukuro, et al. paper in a Letter to the Editor of American Journal of Obstetrics and Gynecology published August 3, 2021:
“Finally, I consider that such an adjustment to EPL risk calculation is not limited to the calculation of the risk for pregnant women with COVID-19. In addition, it should be applied when calculating the EPL to evaluate the impact of COVID-19 vaccination where the period between pregnancy and vaccination is unintentionally excluded.” [Shimabukuro, et al, NEJM, April 21, 2021/October 14, 2021; DOI: 10.1056/NEJMoa2104983, https://www.nejm.org/doi/full/10.1056/NEJMoa2104983.]
Perhaps Sun’s criticism prompted the September 8, 2021, edit of the June 17, 2021, version of Shimabukuro, et al. and the authorless edit of the Riley editorial in the same issue?
The CDC’s Dana M. Meaney-Delman, MD, Sascha R. Ellington, PhD and Tom T. Shimabukuro, MD agreed with Dr. Sun:
“We agree that the denominator used in that proportion — 827 completed pregnancies — is not an appropriate denominator for the calculation of a risk estimate or rate. [https://www.nejm.org/doi/full/10.1056/NEJMc2113516]
In this preliminary report, follow-up information was missing for the majority of pregnancies in which exposure to vaccination occurred in early pregnancy.” [https://www.nejm.org/doi/full/10.1056/NEJMc2113516]
They had 20-week gestational history on only 204 of 1,224 pregnant women receiving at least one injection “before conception” or “in the first trimester.” Before conception? How much before conception? When exactly were these women injected?
They go on to say that of these 1,224 women, they had data only on 204 women through 20 weeks. What do these 204 women with 20 weeks of follow-up have to do with the 104 with spontaneous abortions? Different data queries perhaps?
In the last paragraph of their response to Dr. Sun’s letter, they mentioned completing a telephone survey of the “905 other pregnancies,” and they “enrolled additional persons in the V-safe pregnancy registry.” More added? How many? Of what kind of cases?
Not done yet, they cite the Zauche, et al. “Correspondence” of which Meany-Delman, et al. is a co-author in the same issue of the Journal. In the “Correspondence,” they fail to reference their August 9, 2021, Zauche, et al. preprint paper in Research Square, but they attach a 15-page supplement containing the Research Survey data set and analysis. The third publication, again without peer review, of Zauche, et al. appeared in the October 14, 2021, NEJM along with Meany-Delman, et al. reporting about the same Zauche, et al. August 2021 original report. [https://www.nejm.org/doi/full/10.1056/NEJMc2113891]
Suddenly, one begins to empathize with the World War II bomber crews flying through heavy Triple A. Stealth edits are now joined with stealth publications.
More Zauche, et al.
[https://www.researchsquare.com/article/rs-798175/v1]
Exhibit V presents a summary of the Zauche, et al. paper.
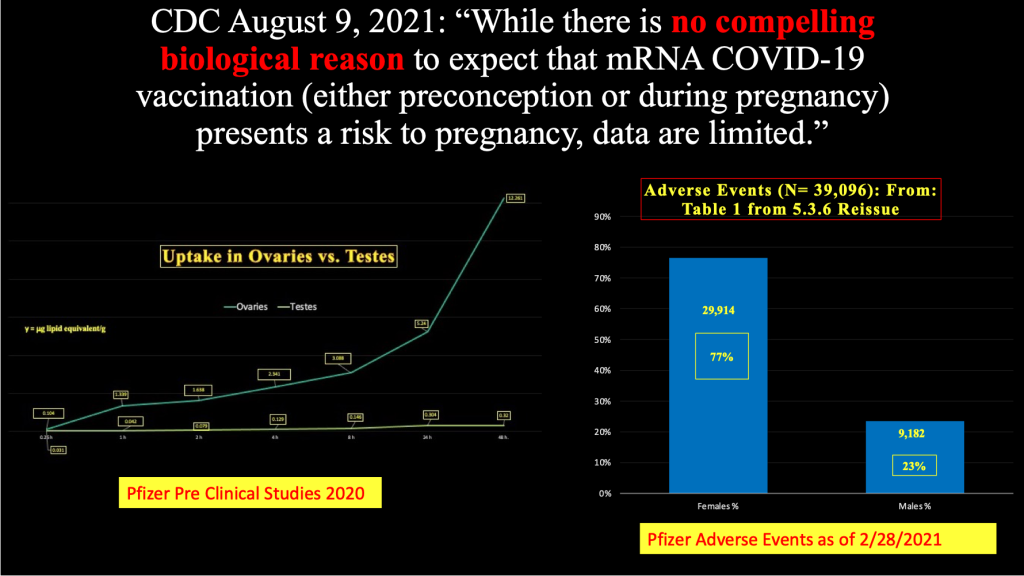
In the August 2021 version of this presentation of data, the authors make a statement similar to that of Dr. Riley – that they knew of no compelling biologic reason why these novel, never-before-used concoctions of two cationic lipids, ALC-0159, ALC-0315, novel messenger ribonucleic acid and other disclosed and undisclosed substances would have a negative impact on pregnant females and their babies.
At the time of this August 2021 declaration, the CDC and FDA had to be aware of the ovarian uptake of LNP/mRNA revealed in Pfizer document “2.4 Nonclinical Overview” [https://www.phmpt.org/wp-content/uploads/2022/03/125742_S1_M2_24_nonclinical-overview.pdf] reporting on pre-clinical studies in Wistar-Han rats in 2020 and the harms to women that had surfaced as of February 28, 2021.
[https://robertchandler.substack.com/p/pfizer-pre-clinical-studies-review, https://robertchandler.substack.com/p/pfizer-document-536-cumulative-analysis, and https://dailyclout.io/women-have-three-times-the-risk-of-adverse-events-than-men-risk-to-the-reproductive-organs-is-even-greater-report/]
Chart 1 presents ovarian uptake of LNP/mRNA in preclinical studies and disproportionate harmful effects of LMP/mRNA in women as of March and April 2021.
Chart 1:
Ovarian uptake of LNP/mRNA from 2020 Pre-Clinical Studies Pfizer Confidential Document 2.4 (left) and Female Predominance in Adverse Events and Adverse Events of Special Interest (right) gathered by Pfizer and reported in Pfizer Confidential Document 5.3.6. [https://www.phmpt.org/wp-content/uploads/2022/03/125742_S1_M2_24_nonclinical-overview.pdf and https://www.phmpt.org/wp-content/uploads/2022/04/reissue_5.3.6-postmarketing-experience.pdf]
A member of the European Medicines Agency (EMA), however, had expressed concern about the lipid component of these products on December 22, 2021:
“According to the product information supplied by the European Medicines Agency, two of the main components of Pfizer’s Comirnaty vaccine are ALC-0315 and ALC-0159. Echelon, the manufacturer of these nanoparticles, specifies that they are ‘for research only and not for human use’. Administering a vaccine – particularly to children – which contains unauthorised (sic) excipients is illegal, dangerous and unethical.
- How does the Commission justify distributing a product that is harmful to public health and, as such, infringes Article 168(1) of the Treaty on the Functioning of the European Union?
- How can it explain such a serious oversight – particularly given that the EU founded a European Health Emergency Preparedness and Response Authority (HERA) in September 2021 – and how will it avoid similar occurrences in future?
- What does it intend to do to put an end to the persistent threat that unauthorised (sic) vaccine components pose to people in Europe?” [https://www.europarl.europa.eu/doceo/document/P-9-2021-005690_EN.html]
During the time from December 14, 2020, until sometime in Spring of 2022, Pfizer had received tens of thousands of Adverse Event (AE) reports concerned with reproductive organ and reproductive function in women. [https://dailyclout.io/women-have-three-times-the-risk-of-adverse-events-than-men-risk-to-the-reproductive-organs-is-even-greater-report/]
Table 1 gives a partial listing of diagnoses and number of reports by diagnostic category. To be fair, this list was published in April 2022. However, this list had been growing steadily all through the first year of widespread distribution and transfection of uncounted millions of pregnant women with LNP/mRNA products.
Table 1
“APPENDIX 2.1 Cumulative Number of Case Reports (Serious and Non-Serious, Medically Confirmed and Non Medically-Confirmed) from Post-Marketing Data Sources, Overall, by Sex, Country, Age Groups and in Special Populations and Summary Tabulation by Preferred Term and MedDRA System Organ Class,” April 16, 2022 [https://www.tga.gov.au/sites/default/files/2022-08/foi-3727-01.pdf]
| Total AEs N = | 923194 |
| Heavy menstrual bleeding | 27685 |
| Menstrual disorder | 22145 |
| Menstruation irregular | 15083 |
| Menstruation delayed | 13989 |
| Dysmenorrhea | 13904 |
| Intermenstrual bleeding | 12424 |
| Amenorrhea | 11363 |
| Polymenorrhea | 9546 |
| Breast pain | 4800 |
| Vaginal hemorrhage | 4699 |
| Oligomenorrhea | 3437 |
| Hypomenorrhea | 2643 |
| Postmenopausal hemorrhage | 2456 |
| Abortion spontaneous | 1809 |
| Breast swelling | 1339 |
| Menstrual discomfort | 1199 |
| Premenstrual syndrome | 998 |
| Breast tenderness | 792 |
| Menometrorrhagia | 632 |
| Adnexa uteri pain | 609 |
| Premenstrual pain | 585 |
| Breast enlargement | 483 |
| Vaginal discharge | 480 |
| Breast discomfort | 443 |
| Mastitis | 392 |
| Ovulation pain | 347 |
| Endometriosis | 337 |
| Menstrual cycle management | 308 |
| Anovulatory cycle | 273 |
| Uterine pain | 270 |
| Abnormal withdrawal bleeding | 265 |
| Uterine hemorrhage | 231 |
| Vulvovaginal pain | 191 |
| Ovulation delayed | 181 |
| Premature baby | 181 |
| Vulvovaginal mycotic infection | 173 |
| Breast cancer | 147 |
| Fetal death | 147 |
| Fetal growth restriction | 124 |
| Vulvovaginal candidiasis | 122 |
| Breast cyst | 115 |
| Genital hemorrhage | 115 |
| Breast edema | 113 |
| Abnormal uterine bleeding | 100 |
| Pelvic venous thrombosis | 98 |
| Labor pain | 95 |
| Uterine leiomyoma | 91 |
| Polycystic ovaries | 82 |
| Breast discharge | 71 |
| Vulvovaginal pruritis | 71 |
| Breast disorder | 68 |
| Uterine contracture during pregnancy | 68 |
| Ectopic pregnancy | 67 |
| Premature labor | 64 |
| Morning sickness | 62 |
| Vaginal infection | 60 |
| Vulvovaginal discomfort | 59 |
| Abortion | 58 |
| Premature menopause | 58 |
| Vulval ulceration | 56 |
| Stillbirth | 56 |
| Vulvovaginal dryness | 54 |
| Coital bleeding | 46 |
| Ovarian cyst rupture | 44 |
| Premature delivery | 44 |
| Endometrial thickening | 42 |
| Genital burning syndrome | 42 |
| Adenomyosis | 41 |
| Breast abscess | 41 |
| Fetal heart rate abnormal | 41 |
| Menarche | 40 |
| Premenstrual headache | 40 |
| Uterine contractions abnormal | 40 |
| Breast induration | 39 |
| Premature rupture of membranes | 37 |
| Uterine polyp | 37 |
| Vulvovaginal swelling | 37 |
| Abortion induced | 36 |
| Uterine inflammation | 36 |
| Vulval hemorrhage | 34 |
| Pelvic inflammatory disease | 33 |
| Pregnancy | 32 |
| Pelvic discomfort | 30 |
| Premature menarche | 27 |
| Premature ovulation | 27 |
| Breast hematoma | 26 |
| Infertility female | 26 |
| Postpartum hemorrhage | 26 |
| Uterine disorder | 26 |
| Pelvic hemorrhage | 25 |
| Noninfective oophoritis | 23 |
| Vaginal ulceration | 23 |
| Dyspareunia | 22 |
| Ovarian disorder | 22 |
| Unintended pregnancy | 22 |
| Vaginal order | 22 |
| Vulvovaginal inflammation | 21 |
| Breast cancer | 20 |
| Breast disorder female | 20 |
| Hemorrhagic ovarian cyst | 20 |
| Placental disorder | 20 |
| Gestational diabetes | 19 |
| Abortion early | 19 |
| Endometrial disorder | 18 |
| Nipple inflammation | 18 |
| Endometrial hyperplasia | 18 |
| Ovarian hemorrhage | 17 |
| Ovarian failure | 16 |
| Vulvovaginal erythema | 16 |
| Ovarian vein thrombosis | 15 |
| Polymenorrhagia | 15 |
| Threatened labor | 14 |
| Fibrocystic breast disease | 13 |
| Ovarian enlargement | 13 |
| Uterine enlargement | 13 |
| Cervix hemorrhage uterine | 12 |
| Breast atrophy | 11 |
| Breast hemorrhage | 11 |
| Breast neoplasm | 11 |
| Caesarean section | 11 |
| Cervical dysplasia | 11 |
| Pelvic girdle pain | 11 |
| Vaginal disorder | 11 |
| Vulval disorder | 11 |
| Bartholin’s cyst | 10 |
| Decidual cyst | 10 |
| Fetal cardiac disorder | 10 |
| Fetal growth abnormality | 10 |
| Fetal vascular malperfusion | 10 |
| Vaginal cyst | 10 |
| Small for dates baby | 10 |
| Vaginal cyst | 10 |
Criticisms of Zauche, et al.
- Nonrandom sample.
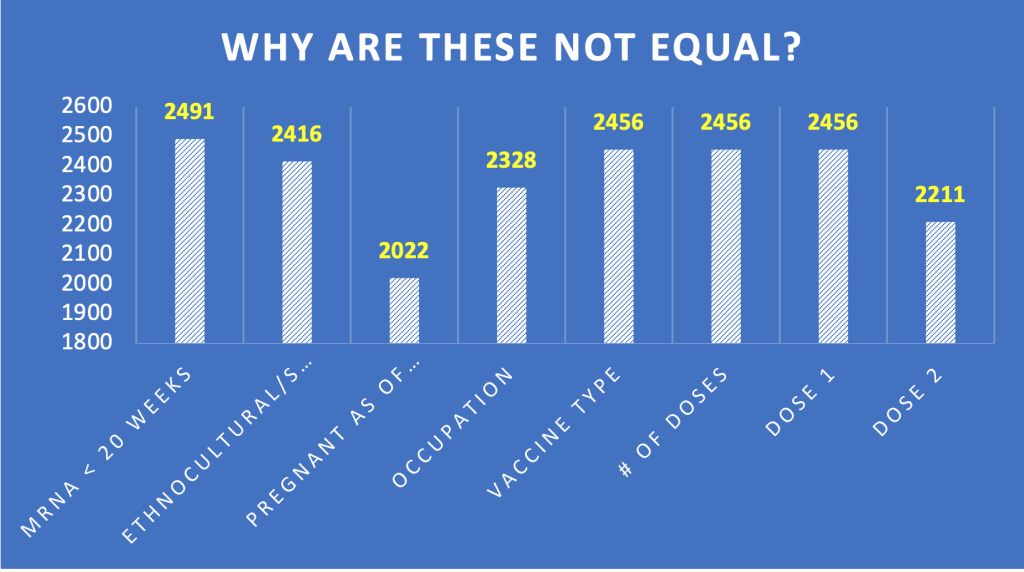
The data set reported by Zauche, et al. was far from a random and representative sample of pregnant women. In fact, 80 percent were white (1,923/2,416) and 94 percent were healthcare workers.
Mukherjee, et al. examined the miscarriage rate in whites versus black and found:
“Our primary finding was that black women have a nearly 2-fold higher risk of miscarriage compared with white women during gestational weeks 10–20, while there was no apparent difference in the risk of earlier miscarriage.” [https://academic.oup.com/aje/article/177/11/1271/97504]
Another curious feature of this sample is that each category has a different number of subjects. We need to know how this happened. Were each of these categories a separate database query?
- Only 1073 or 49 percent of pregnant women received two doses during preconception or first trimester.
This group has no associated data for spontaneous abortions. Demographics, comorbidities and number of spontaneous abortions for this group was not provided. In the August 9, 2021, version of Zauche, et al. in Research Survey, readers were allowed to comment. Robert Clark made the following comment:
Robert Clark Comment on article
on 20 Aug, 2021
“A key flaw in the study is it looked at the average number of SAB’s after at least one dose. It is well-known the 2nd dose is the more injurious one, in terms of side effects. By including also those who had only one dose, you decrease the size of the effect.” [https://www.researchsquare.com/article/rs-798175/v1]
Mr. Clark was on point as the Zauche, et al. data shown in Chart 2 reveals.
Chart 2: The Most Important Study Group
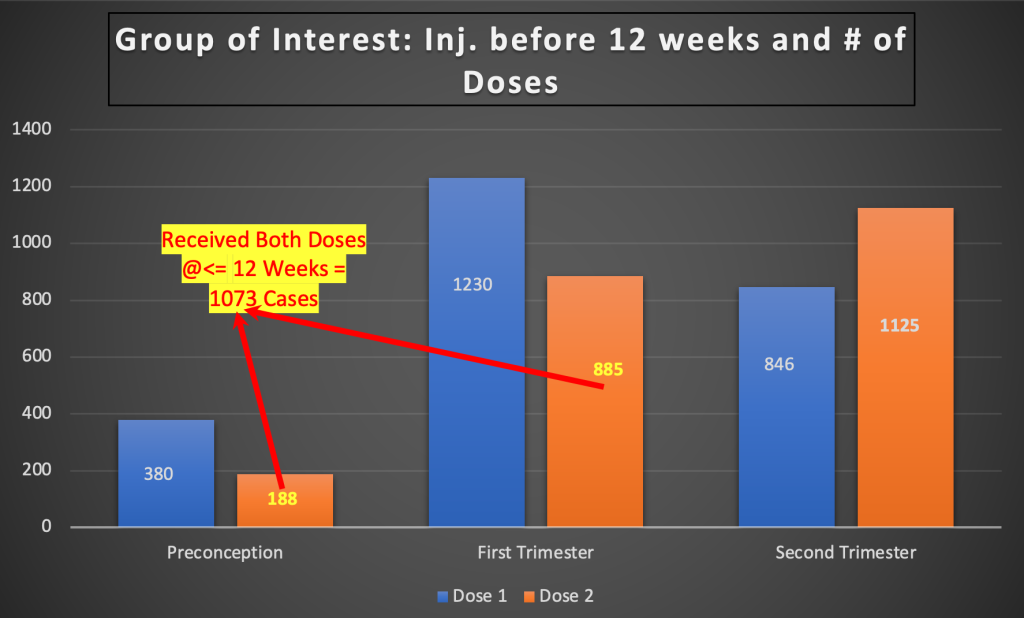
Outcome of pregnancy for these 1,073 women should have been reported as the single most meaningful subgroup. It was not done.
- Omission of first six weeks data.
Zauche, et al. excluded subjects who miscarried during the first six gestational weeks:
“The inclusion of participants pregnant at 6 completed weeks’ gestation reflects when pregnancies are generally recognized and is consistent with previous literature estimating SAB in the general population.5, 8–10, 15”
This remarkable quote misstates the literature. For example, Goldhaber and Fireman found that the more sensitive the testing, the more the frequency of miscarriage in the first six weeks rises.
a. “The fetal life table revisited: spontaneous abortion rates in three Kaiser Permanente cohorts”:
“The major difference in survival between the three Kaiser Permanente cohorts was in the earliest gestational week of observation, week 5 from the last menstrual period, where the older data were sparse and potentially biased. High loss rates during this week accounted for one-fourth to one-third of the cumulative risk observed in the older studies.”
“Because of improved reliability of early pregnancy testing and an emphasis on early prenatal care, the mean gestational age at entry to the 1981-1982 cohort was 10.4 weeks from the last menstrual period compared to 14.3 weeks and 13.7 weeks for the older studies. All three studies showed a peak for risk of spontaneous abortion around weeks 10-12 from the last menstrual period.”
[Goldhaber, M. K., & Fireman, B. H. (1991). The fetal life table revisited: spontaneous abortion rates in three Kaiser Permanente cohorts. Epidemiology (Cambridge, Mass.), 2(1), 33–39. https://pubmed.ncbi.nlm.nih.gov/2021664/]
This finding reinforces a conclusion reported in an earlier study by Wilcox, et al. in which they found that the more carefully they looked for spontaneous abortion in the first six weeks the more miscarriages they identified.
b. “Incidence of Early Loss of Pregnancy”
- Allen J. Wilcox, M.D., Ph.D.,
- Clarice R. Weinberg, Ph.D.,
- John F. O’Connor, Ph.D.,
- Donna D. Baird, Ph.D.,
- John P. Schlatterer, M.S.,
- Robert E. Canfield, M.D.,
- Glenn Armstrong, Ph.D.,
- and Bruce C. Nisula, M.D.
“We identified 198 pregnancies by an increase in the hCG level near the expected time of implantation. Of these, 22 percent ended before pregnancy was detected clinically. Most of these early pregnancy losses would not have been detectable by the less sensitive assays for hCG used in earlier studies.
The total rate of pregnancy loss after implantation, including clinically recognized spontaneous abortions, was 31 percent. Most of the 40 women with unrecognized early pregnancy losses had normal fertility, since 95 percent of them subsequently became clinically pregnant within two years.”
[Wilcox, A. J., Weinberg, C. R., O’Connor, J. F., Baird, D. D., Schlatterer, J. P., Canfield, R. E., Armstrong, E. G., & Nisula, B. C. (1988). Incidence of early loss of pregnancy. The New England Journal of Medicine, 319(4), 189–194. https://pubmed.ncbi.nlm.nih.gov/3393170/]
With the first-time use of a novel, genetically active drug never tested in pregnant women, the best science should have been employed to look particularly closely at spontaneous abortion in the first six weeks. The same is true for preterm births, congenital deformities, complicated deliveries, placental anomalies, and neonatal death. This was simply not done.
- Missing data.
Zauche, et al. report:
“Enrolled participants receive a telephone follow-up each trimester, during the postpartum period, and three months following live births.”
However, two of the 19 comments entered in the “Comments” section by readers of the August 2021 Research Square preprint wrote about having had no follow-up after enrolling in the V-safe Pregnancy Registry:
Dani K on 20 Aug, 2021
“I was a part of the v safe registry. I received the vaccine 32 days prior to becoming pregnant. I reported my pregnancy to the v safe registry 3 times, and was told someone would contact me each time. No one ever did. I subsequently miscarried at 10 weeks. My miscarriage was not counted in this study. Who else’s miscarriage or adverse pregnancy outcome was left out? While I do not personally believe the vaccine caused my miscarriage, one has to wonder about the accuracy of this data.”
Shaena Kauffman on 15 Aug, 2021
“I find this study confusing. I registered in the v-safe program and never got one phone call, only text update requests. I repeated a spontaneous miscarriage which occurred in my 2nd trimester, 2 weeks after my 2nd Covid dose. I reported this. No one contacted me. This data shows only 11 SAB. I highly doubt it is counting me. Again, I reported all the ways you can. I got my VARES [sic] ID. Not one call. Is it counting events reported in the database? If you search you will see far more than 11 reports. Additional clarification on the data is needed.”
[https://www.researchsquare.com/article/rs-798175/v1]
Perhaps this is how you capture the data for a tiny nonrepresentative sample of the hundreds of thousands of pregnant women who were transfected with LNP/mRNA gene therapy products?
- Data is not stratified.
A proper study of the complex subject of adverse effects on the human reproductive cycle should include stratification in adequately powered samples. What drug was administered, what were the batch numbers, dates of administration relative to gestation, age, comorbidities and relevant demographic diversity are important. The V-safe Pregnancy Registry contained little of this data.
- Sample size is small.
Only 1,073 preconception or first trimester pregnant women were given both doses. Demographics, spontaneous abortion numbers, and outcomes are missing for this critical group.
By this point in time, millions of pregnant women had been given LNP/mRNA products. A very small, nonrandom sample is likely to provide only incorrect and or unusable data.
- Pregnancy outcome data not provided.
Zauche, et al. did not have outcome data on the cases they presented.
Shimabukuro, et al., December 2021 Versions
[https://journals.lww.com/obgynsurvey/Abstract/2021/12000/Preliminary_Findings_of_mRNA_COVID_19_Vaccine.7.aspx and https://journals.lww.com/obstetricanesthesia/Abstract/2021/12000/Preliminary_Findings_of_mRNA_Covid_19_Vaccine.2.aspx]
The 12 months following widespread injection of LNP/mRNA gene therapy products in pregnant women saw a reiteration of the calculation of Total Fetal Loss figure of 13.6 percent or 115/827 in two final publications by the government health agencies. Both publications were in the Ob Gyn literature, Exhibit VI. [https://journals.lww.com/obgynsurvey/Abstract/2021/12000/Preliminary_Findings_of_mRNA_COVID_19_Vaccine.7.aspx and https://journals.lww.com/obstetricanesthesia/Abstract/2021/12000/Preliminary_Findings_of_mRNA_Covid_19_Vaccine.2.aspx]
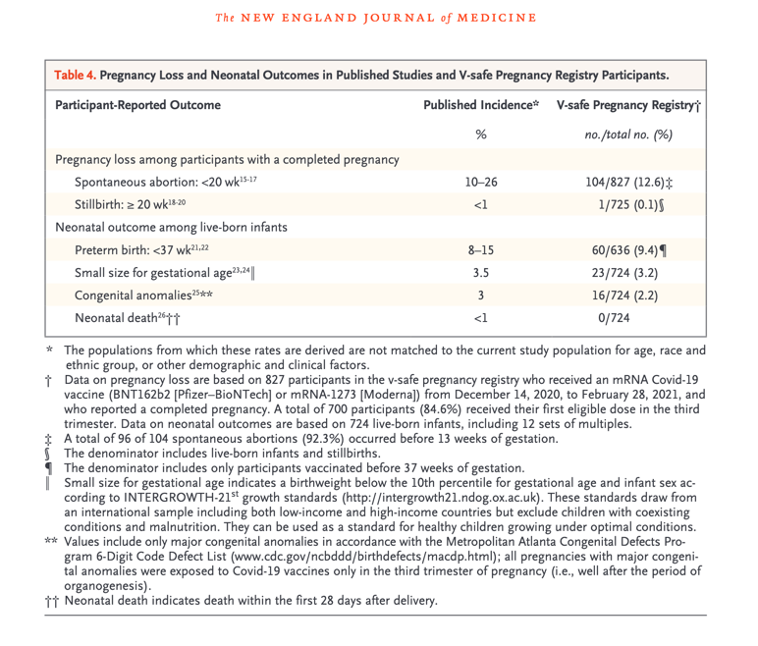
The year ended much as it had begun, except for the correction of Table 4 in the original April 21, 2021, original version of Shimabukuro, et al.
No denominator exists to calculate the rate of spontaneous abortion in pregnant women injected with LNP/mRNA experimental genetic material. Exhibit VII.
Further Studies 2021-2022: Clinical Trials Notation
[https://clinicaltrials.gov/ct2/show/NCT04754594?term=BNT162b2&draw=2&rank=10]
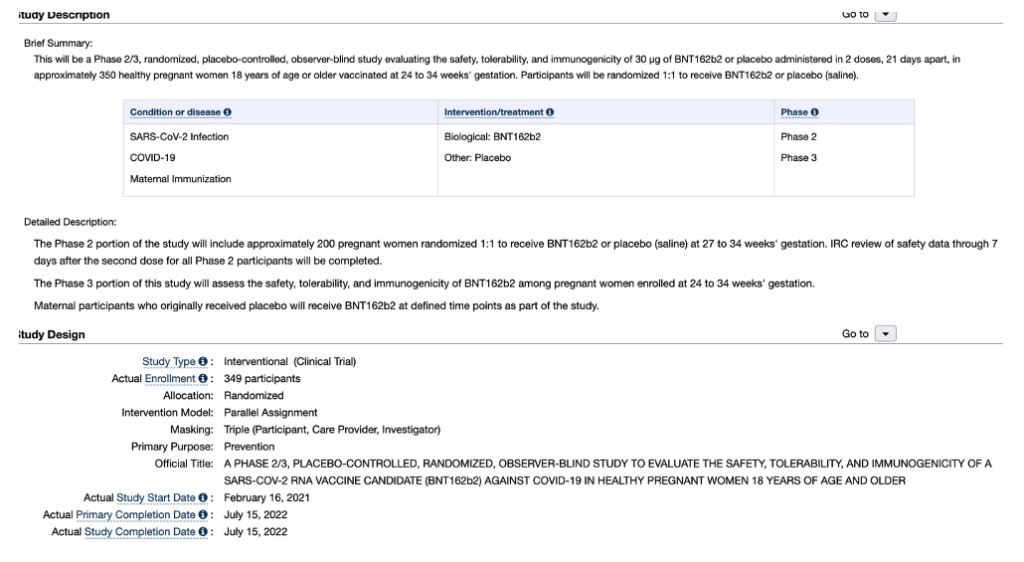
On July 15, 2022, there was a notice on ClinicalTrials.gov concerning completion of a randomized, placebo-controlled, observer-blind study of safety, tolerability, and immunogenicity of two doses, 21 days apart, in third trimester pregnant women. Exhibit VIII.
To date no results have been released from this study.
“However, only women in the third trimester were recruited for this study.” Unfortunately, it is the first trimester about which it is vital to have data. Why do a study of pregnant women given BNT162b2 during gestational weeks 27-34?
Obfuscation
The essence of the CDC/FDA reporting in the first 12 months follow-up of 35,691 pregnant women entered into the V-safe data base boils down to known outcomes in 827. This could have been summarized in the final version of Table 4 in the June 17, 2021, version of Shimabukuro, et al. [https://www.researchsquare.com/article/rs-798175/v1] Tables 1-3 and Chart 1 are presented in Exhibit IX for reference.
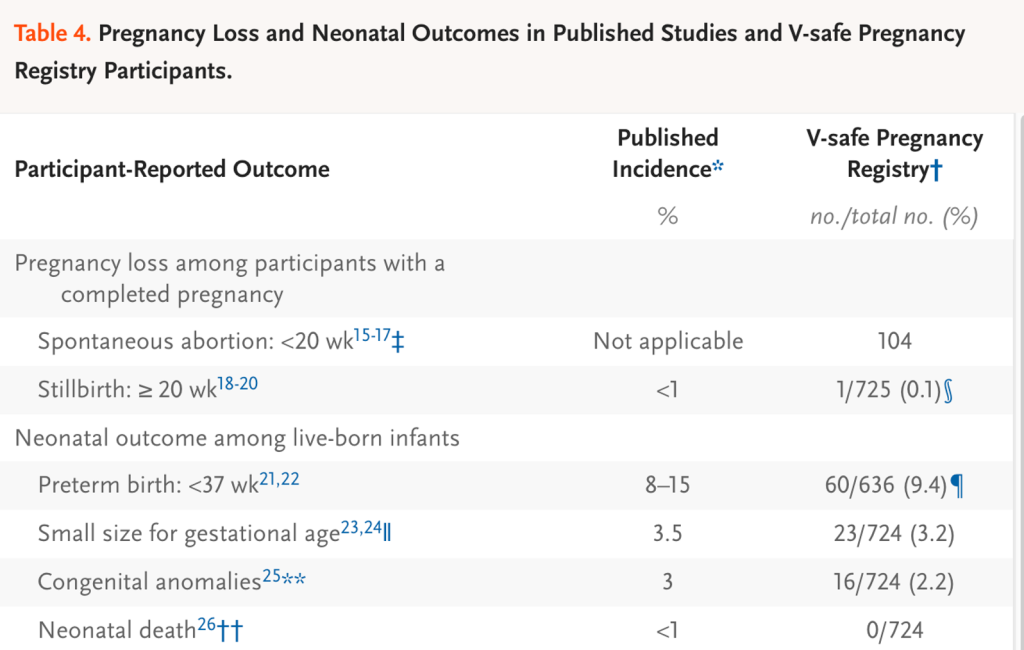
There is little of value in the rest of the Shimabukuro et al. paper in its various versions, as well as its progeny; but the reader must fight through a sizeable smokescreen of various data sets with no outcome. We will examine this smokescreen in some detail.
Spontaneous abortion:
The double sword footnote in the table above informs the reader that there is no suitable denominator for the 104 spontaneous abortions, so a rate of abortion cannot be determined.

Stillbirth:
A stillbirth was reported to have occurred in 1 of 725 or 0.1% of some unknown “group.” However, the gestational age at the time of injection was not spelled out. To be meaningful, this data needs to be stratified by trimester, Moderna versus Pfizer, mothers’ ages, prior gestational history, comorbidities. We do know that only 127 mothers were injected in the first or second trimester and were followed to completion. Here is the footnote for stillbirths:

Preterm birth:
Preterm births occurred 60 of 636 pregnancies. The origin of this denominator of 636 is not provided. The footnote for this entry is not much help in revealing the origin of the 636 figure, but we do learn that all three trimesters were included. As in the case of stillbirth, many relevant parameters are absent.

Small size for gestational age:
The denominator here is 724 or the same as the denominator given above under stillbirths (725) minus the one stillbirth. This pattern is repeated for congenital anomalies and neonatal death.
The matter to debate here is whether any of these numbers are valid and reliable estimates of rates of spontaneous abortion, stillbirth, preterm birth, congenital anomalies, and neonatal death as none of the denominators are reliable indicators of what happened to a representative large sample of mothers injected with LNP/mRNA products during their first term.
What is provided in Tables 1-3 and a single chart are the following:
- Table 1: Demographic data on 35,691 pregnant women receiving LNP/mRNA injections.
- Table 2: Reactogenicity data from four subgroups, Pfizer 1 N = 9,052, Pfizer 2 N = 6,638, Moderna 1 N = 7,930 and Moderna 2 N = 5,635.
- Figure 1: A plot of the reactogenicity data from an unspecified group other than they completed a day 1 survey.
- Table 3: Age brackets, race and ethnic identity, timing of the first eligible dose, and incidence of Covid-19 during pregnancy for various non-identified subsets of 3,958 registrants in the pregnancy Registry.
- Tables 1-3 combined with Chart 1 have no value informing the reader as to how often spontaneous abortion, stillbirth, preterm birth, small size for gestational age, congenital deformity and neonatal death occur after first trimester inoculation with LNP/mRNA gene therapy products.
These large Tables and complex Charts may blunt the senses of some readers and obscure the shortcomings of the post EUA surveillance efforts by government health agencies.
Conclusions:
Remarkably, after approximately one year and the efforts of:
- 21 authors and 47 members of the CDC Covid-19 Response V-safe Pregnancy Registry Team in 22 divisions of the CDC and FDA reporting in Shimabukuro, et al.
- 13 authors, 59 members of the CDC Covid-19 Response V-safe Pregnancy Registry Team, now joined with colleagues from NIH, the US Department of Energy, the US Public Health Service, the National Institute for Occupational Health and Safety and the National institute of Environmental Health Sciences in Zauche, et al. Exhibit X.
- $13,922,163,000 in taxpayer money. [https://www.cdc.gov/budget/documents/fy2021/FY-2021-CDC-Operating-Plan.pdf and https://www.fda.gov/media/149526/download]
reliable and valid outcome data concerning the safety of LNP/mRNA experimental gene products in hundreds of thousands and perhaps millions of pregnant women and their babies was not produced.
Furthermore, future reporting by these individuals or others from these agencies should not be accepted without access to raw data and complete description of the exact methodology used to obtain it.
Exhibits
Exhibit I: Tracking CDC and FDA Publications in Calendar Year 2021
Exhibit II: Shimabukuro, et al. Publication Dates and Edits
- When was this study first published?
A. Below is the first page of the June 17, 2021, version of Shimabukuro, et al. indicating a publication date of April 21, 2021, at NEJM.org. However, attempts to access the April 21, 2021, version online returns the June 17, 2021, version. A hard copy version of the NEJM article does exist.

Above: a recent online search for the April 21? April 22? Article returns an article with the June 17, 2021, publication date.
B. There is no April 21, 2021, publication date listed in the NEJM Online Index. The closest date is April 22, 2021.
C. Online search for Table of Contents for April 21 or 22, 2021, lists no such article:
https://www.nejm.org/toc/nejm/384/15
Here it is in hard copy downloaded shortly after publication date April 21 or 22, 2021.

D. April 21, June 17, September 8, October 14 – all 2021 – NEJM versions.
A hard copy of the April 21, 2021, report appears to be the original version of the June 17, 2021, publication. Currently, the June 17, 2021, version online was modified on September 8, 2021, but no online version for that date is currently available. The October 14, 2021, edition of NEJM acknowledges the September 8, 2021, revisions in the June 17, 2021, republication of the April 21, 2021, original.
E. September 8, 2021, corrections of the April 21, 2021, original Shimabukuro, et al. paper, republished online June 17, 2021, as reported in the October 14, 2021, online NEJM are presented below.
Author: No Author Listed dated October 14, 2021,
N Engl J Med 2021; 385:1536
DOI: 10.1056/NEJMx210016
- p. 2273 third sentence of the Abstract (Actually, the quote was in the fourth sentence.)
Correction:
“Among 3958 participants enrolled in the v-safe pregnancy registry, 827 had a completed pregnancy, of which 115 (13.9%) were pregnancy losses and 712 (86.1%) were live births (mostly among participants vaccinated in the third trimester).”
Original:
rather than “…of which 115 (13.9%) resulted in a pregnancy loss and 712 (86.1%) resulted in a live birth (mostly among participants with vaccination in the third trimester).”
- Discussion section (p. 2277), the parenthetical in the third sentence should have begun,
Correction:
“(i.e., preterm birth, small size, …,”
Original:
“(e.g., fetal loss, preterm birth, small size, ….”
- Table 4 (p. 2280)
Corrections:
Spontaneous abortion: <20 wk15-17‡ current Table 4 after revisions.
The “Published Incidence” cell in the same row should have read
“Not applicable,” rather than “10–26,” and the
“V-safe Pregnancy Registry” cell should have read “104,” rather than “104/827 (12.6)‡.”
4. Double sword footnote on p. 2280 was added.

5. In the Table 4 footnotes, the following content was next to the double dagger footnote:
“No denominator was available to calculate a risk estimate for spontaneous abortions,
because at the time of this report
follow-up through 20 weeks was not yet available for 905 of the 1224 participants
vaccinated within 30 days before the first day of the last menstrual period or in the first trimester.
Furthermore, any risk estimate would need to account for gestational week–specific risk of spontaneous abortion.”
“Updates” in June, September, October and December of 2021 provided no new information about the 35,691 pregnant women injected with LNP/mRNA in December 2020 thru February 2021 in V-safe or the 3958 injected pregnant women in the Pregnancy Registry. There should have been completion of pregnancy data on the pregnant women injected in first 6 weeks by October and all of 10 weeks by December 2021 yet the December update (Obstetrical & Gynecological Survey, December 2021, 76, 12, 729-731) reported on only 827 completed pregnancies.
F. Current Status as of September 6, 2022
Shimabukuro, et al. September 6, 2022, version of the June 17, 2021, publication (downloaded August 22, 2022, and checked again on September 6, 2022)
A. Abstract in the September 6, 2022, version, page 2273:
“Among 3958 participants enrolled in the v-safe pregnancy registry, 827 had a completed pregnancy, of which 115 (13.9%) were pregnancy losses and 712 (86.1%) were live births (mostly among participants vaccinated in the third trimester).”
The errors in this calculation are explained below:
- Numerator = 115 = 104 Spontaneous Abortions (< 20 weeks) + 1 Stillbirth (>20 weeks) + 10 medical abortions.
- Denominator = 827 = 127 first and second trimester cases + 700 third trimester cases
B. Text on p. 2276 in the September 6, 2022, version:
“Among 827 participants who had a completed pregnancy, the pregnancy resulted in a live birth in 712 (86.1%), in a spontaneous abortion in 104 (12.6%), in stillbirth in 1 (0.1%), and in other outcomes (induced abortion and ectopic pregnancy) in 10 (1.2%).”
The errors in this calculation are explained below:
- Numerator = 104 Spontaneous Abortions (less than 20 weeks)
- Denominator = 827 = 700 stillbirths in third trimester cases and 127 first and second trimester cases (spontaneous abortions – i.e., <20 weeks’ gestation – plus stillbirths for >20 weeks in second trimester)
Table 4 double sword footnote, p. 2280:
“A total of 96 of 104 spontaneous abortions (92.3%) occurred before 13 weeks of gestation. No denominator was available to calculate a risk estimate for spontaneous abortions, because at the time of this report, follow-up through 20 weeks was not yet available for 905 of the 1224 participants vaccinated within 30 days before the first day of the last menstrual period or in the first trimester. Furthermore, any risk estimate would need to account for gestational week–specific risk of spontaneous abortion.”
The corrections that currently exist in the June 17, 2021, version of the Shimabukuro, et al. document having been adjusted retroactively such that a reader today would not know the calculation of Spontaneous Abortion Rate had been dropped in a September 8, 2021, revision of the June 17, 2021, republication of the original April 21, 2021, document.
Exhibit III. Riley.
Editorial.
“mRNA Covid-19 Vaccines in Pregnant Women”
Laura E. Riley, MD,
N Engl J Med 2021; 384:2342-2343
DOI: 10.1056/NEJMe2107070
[https://www.nejm.org/doi/full/10.1056/NEJMe2107070]
Dr. Riley’s editorial discussed the Shimabukuro, et al. paper that curiously appears in the same issue of NEJM as the Shimabukuro, et al. article itself. Was she responding to the April 2021 publication?
This editorial was published on June 17, 2021. There were then two versions of the June 17, 2021, Shimabukuro et al. paper – the original and a version that was revised on September 8, 2021. Notification about the revision was made in the October 14, 2021, issue of NEJM.
Dr. Riley’s bio:
“Laura E. Riley, MD, a renowned obstetrician who specializes in obstetric infectious disease, has been appointed Chair of the Department of Obstetrics and Gynecology at Weill Cornell Medicine and Obstetrician and Gynecologist-in-Chief at New York-Presbyterian/Weill Cornell Medical Center.” [https://www.nyp.org/publications/professional-advances/gynecology/dr-laura-e-riley-new-chair-of-obstetrics-and]
Dr. Riley is a member of the Editorial Board of the New England Journal of Medicine.

Dr. Riley made the following statement in her editorial:
“It is notable that as of April 26, 2021, more than 100,000 pregnant women reported having received a Covid-19 vaccination and yet only a small fraction (4.7%) have enrolled in the v-safe pregnancy registry.” [https://www.nejm.org/doi/full/10.1056/NEJMe2107070]
Corrections of Dr. Riley’s editorial dated September 8, 2021, were reported in the October 14, 2021, issue of NEJM. [https://pubmed.ncbi.nlm.nih.gov/34496193/ and https://www.nejm.org/doi/10.1056/NEJMx210017?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed]
mRNA Covid-19 Vaccines in Pregnant Women.
[No authors listed]
N Engl J Med. 2021 Oct 14;385(16):1536. doi: 10.1056/NEJMx210017. Epub 2021 Sep 8. PMID: 34496193
No abstract available.
Corrections to the June 17, 2021, Riley editorial:
Original June 17, 2021:
“Among 827 registry participants who reported a completed pregnancy, the pregnancy resulted in a spontaneous abortion in 104 (12.6%) and in stillbirth in 1 (0.1%); these percentages are well within the range expected as an outcome for this age group of persons whose other underlying medical conditions are unknown.”
Revision #1
In the Results section of the Abstract (page 2273), the third sentence should have read:
“Among 827 registry participants who reported a completed pregnancy, 104 experienced spontaneous abortions and 1 had a stillbirth,”
rather than,
“…a completed pregnancy, the pregnancy resulted in a spontaneous abortion in 104 (12.6%) and in stillbirth in 1 (0.1%); these percentages are well within the range expected as an outcome for this age group of persons whose other underlying medical conditions are unknown.”
Revision #2
In the first paragraph of the “Discussion” section (page 2277), the parenthetical in the third sentence should have begun:
“(i.e., preterm birth, small size, …,”
rather than
“(e.g., fetal loss, preterm birth, small size, ….”
Revision #3
A. In Table 4 (page 2280), the double dagger symbol in the “Spontaneous abortion” row should have followed:
“Spontaneous abortion: <20 wk15-17.”
Actual
“Spontaneous abortion: <20 wk15-17 ‡”
B. The “Published Incidence” cell in the same row should have read “Not applicable,” rather than “10–26,”
Actual
“Not applicable”
C. “V-safe Pregnancy Registry” cell should have read “104,”
rather than “104/827 (12.6) ‡.”
Actual
104
Revision #4
In the table footnotes, the following content should have been appended to the double dagger footnote:
“No denominator was available to calculate a risk estimate for spontaneous abortions, because at the time of this report, follow-up through 20 weeks was not yet available for 905 of the 1224 participants vaccinated within 30 days before the first day of the last menstrual period or in the first trimester. Furthermore, any risk estimate would need to account for gestational week–specific risk of spontaneous abortion.”
Actual:
“No denominator was available to calculate a risk estimate for spontaneous abortions, because at the time of this report, follow-up through 20 weeks was not yet available for 905 of the 1224 participants vaccinated within 30 days before the first day of the last menstrual period or in the first trimester. Furthermore, any risk estimate would need to account for gestational week–specific risk of spontaneous abortion.”
“The article is correct at NEJM.org.”
Exhibit IV: Hong Sun, PhD Correspondence, NEJM October 14, 2021.
Criticism
Dr. Hong Sun, PhD of Antwerp, Belgium presented his objections to the calculated rate of spontaneous abortions in the June 17,2021, version. He spotted the error made in using the wrong denominator.
“As stated in the article, among the 827 participants with a completed pregnancy, 700 received their first eligible vaccine dose in the third trimester. These participants should be excluded from the calculation because they had already passed week 20 when they received the vaccination. The risk of spontaneous abortion should be determined on the basis of the group of participants who received the vaccination before week 20 and were followed through week 20 or had an earlier pregnancy loss.”
This letter was reportedly published on September 8, 2021, at NEJM.org.
Response of Dr. Dana M. Meaney-Delman, MD, et al. in the same October 14, 2021, issue of NEJM.
The authors’ reply:
“Sun appropriately raises questions about the proportion of women reporting spontaneous abortion in our recent article. We agree that the denominator used in that proportion — 827 completed pregnancies — is not an appropriate denominator for the calculation of a risk estimate or rate.
The number of spontaneous abortions (104) reflects data reported by the participants as of March 30, 2021, during telephone follow-up. In this preliminary report, follow-up information was missing for the majority of pregnancies in which exposure to vaccination occurred in early pregnancy.
Among the 1224 women who had been vaccinated before conception or in the first trimester, follow-up through 20 weeks of gestation had been completed for only 204 pregnancies that were known to be ongoing and for 1 pregnancy that resulted in stillbirth.
Among the pregnancies that had not yet reached 20 weeks of gestation, there were 10 pregnancies with other outcomes before 20 weeks of gestation, including 8 ectopic pregnancies and 2 induced abortions.
For the other 905 pregnancies, follow-up had not occurred to establish whether these pregnancies were ongoing past 20 weeks of gestation.
We have amended Table 4 in our earlier publication and have clarified the text.
Subsequently, we completed telephone follow-up for the 905 pregnancies and enrolled additional persons in the v-safe pregnancy registry.
To determine the cumulative risk of spontaneous abortion from 6 to less than 20 weeks of gestation, we used life-table methods to perform an updated analysis, now reported in the Journal, involving 2456 women who received at least one dose of an mRNA Covid-19 vaccine before conception or before 20 weeks of gestation.1
The estimated risks (14.1% overall and 12.8% in age-standardized analyses) are consistent with the risks of spontaneous abortion reported in the general population.1
Dana M. Meaney-Delman, M.D.
Sascha R. Ellington, Ph.D.
Tom T. Shimabukuro, M.D.
Centers for Disease Control and Prevention, Atlanta, GA
tshimabukuro@cdc.gov”
This letter was published on September 8, 2021, at NEJM.org.
Zauche LH, Wallace B, Smoots AN, et al. Receipt of mRNA Covid-19 vaccines and risk of spontaneous abortion. N Engl J Med 2021;385:1533-1535.
Exhibit V. Zauche, et al. Receipt of mRNA Covid-19 Vaccines and Risk of Spontaneous Abortion
October 14, 2021
N Engl J Med 2021; 385:1533-1535
DOI: 10.1056/NEJMc2113891
[https://www.nejm.org/toc/nejm/385/16?query=article_issue_link]
Lauren H. Zauche, Ph.D., M.S.N.
Bailey Wallace, M.P.H.
Ashley N. Smoots, M.P.H.
Christine K. Olson, M.D., M.P.H.
Titilope Oduyebo, M.D., M.P.H.
Shin Y. Kim, M.P.H.
Emily E. Petersen, M.D.
Jun Ju, M.S.
Jennifer Beauregard, Ph.D., M.P.H.
Centers for Disease Control and Prevention (CDC), Atlanta, GA
Allen J. Wilcox, M.D., Ph.D.
National Institutes of Health, Durham, NC
Charles E. Rose, Ph.D.
Dana M. Meaney-Delman, M.D., M.P.H.
Sascha R. Ellington, Ph.D., M.S.P.H.
CDC, Atlanta, GA
Editor’s Note: This letter reportedly was published on September 8, 2021, at NEJM.org according to an editor’s note at the top of the October 14, 2021, publication. There is no such paper listed at NEJM.org for September 8, 2021.

NEJM Volume 385 No. 11 dated September 9, 2021, has no such article, https://www.nejm.org/toc/nejm/385/11.

This two-and-a-half-page note appeared under the heading of “Correspondence” in the October 14, 2021, edition of the NEJM.
This report is an updated reporting of the CDC V-safe registry to determine the cumulative risk of spontaneous abortion from 6 to less than 20 weeks of gestation.
The authors’ note:
“Although spontaneous abortion (pregnancy loss occurring at less than 20 weeks of gestation) is a common pregnancy outcome affecting 11 to 22% of recognized pregnancies (see Table S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org),2-4 data to inform estimates of the risk of spontaneous abortion after receipt of an mRNA Covid-19 vaccine either before conception (30 days before the first day of the last menstrual period through 14 days after) or during pregnancy are limited.” (Paragraph 1.)
The analysis included singleton pregnancies who received one dose of an mRNA vaccine before conception or before 20 weeks of gestation and who did not have a pregnancy loss before six weeks of gestation.
The second paragraph describes what the authors refer to use of “life table methods” to calculate the risk of spontaneous abortion. What is meant by this is not specified in the text other than a reference from the British Medical Journal. Magnus MC, Wilcox AJ, Morken N-H, Weinberg CR, Håberg SE. Role of maternal age and pregnancy history in risk of miscarriage: prospective registry based study. BMJ 2019;364: l869-l869.
“Life table methods were used to calculate the cumulative risk of spontaneous abortion according to gestational week, with appropriate left truncation (i.e., with adjustment for gestational age at entry); data were right-censored at the time of the most recent contact for participants with ongoing pregnancies who were not contacted at 20 weeks of gestation or later and at the time of the outcome for participants who reported pregnancy outcomes other than spontaneous abortion (induced abortions or ectopic or molar pregnancies) before 20 weeks of gestation.”
“A total of 2456 participants who were enrolled in the CDC v-safe Covid-19 pregnancy registry met the inclusion criteria for this study;
- 2022 participants reported ongoing pregnancies at 20 weeks of gestation,
- 165 participants reported a spontaneous abortion
- (154 participants before 14 weeks of gestation),
- 65 participants with most recent contact during the first trimester could not be reached for second trimester follow-up,
- 188 participants completed second trimester follow-up before 20 weeks of gestation,
- 16 participants reported another pregnancy outcome before 20 weeks (induced abortion or ectopic or molar pregnancy) (Fig. S1).
- Most participants were 30 years of age or older (77.3%), were non-Hispanic White (78.3%), and worked as health care personnel (88.8%).
- Slightly more than half the participants (52.7%) had received the BNT162b2 vaccine (Pfizer–BioNTech) (Table S2).
- The cumulative risk of spontaneous abortion from 6 to less than 20 weeks of gestation was 14.1% (95% confidence interval [CI], 12.1 to 16.1) in the primary analysis (Table 1) and 12.8% (95% CI, 10.8 to 14.8) in an analysis using direct maternal age–standardization to the reference population.
- The cumulative risk of spontaneous abortion increased with maternal age (Table S3). In the sensitivity analysis, under the extreme assumption that all 65 participants with most recent contact during the first trimester had a spontaneous abortion, the cumulative risk of spontaneous abortion from 6 to less than 20 weeks of gestation was 18.8% (95% CI, 16.6 to 20.9); after age standardization, the cumulative risk was 18.5% (95% CI, 16.1 to 20.8).”
This perplexing analysis is presented in more detail in the Supplementary Appendix.
Curiously, this analysis was not cited in the December 2021 version of Shimabukuro, et al. [https://journals.lww.com/obgynsurvey/Abstract/2021/12000/Preliminary_Findings_of_mRNA_COVID_19_Vaccine.7.aspx]
Exhibit VI. Shimabukuro, et al. Final Report for 2021.
Obstetrical & Gynecological Survey
December 2021 | Volume 76 | Issue 12 | pp: 729-731
doi: 10.1097/01.ogx.0000802676.57373.17
[https://journals.lww.com/obgynsurvey/Fulltext/2021/12000/Preliminary_Findings_of_mRNA_COVID_19_Vaccine.7.aspx]
OBSTETRICS: MEDICAL COMPLICATIONS OF PREGNANCY
Preliminary Findings of mRNA COVID-19 Vaccine Safety in Pregnant Persons
Tom T. Shimabukuro
“Overall, 92 (2.3%) of participants received their first vaccination dose during the preconception period, 1132 (28.6%) in the first trimester, 1714 (43.3%) in the second trimester, and 1019 (25.7%) in the third trimester. In terms of adverse effects, injection site pain was described more among pregnant persons compared with nonpregnant women.
Headache, myalgia, chills, and fever were reported less often among pregnant persons compared with nonpregnant people. Of the 3958 participants enrolled in the v-safe pregnancy registry, 827 had a completed pregnancy. Of these, 827 completed pregnancies, 115 (13.9%) resulted in a pregnancy loss, and 712 (86.1%) resulted in a live birth (mainly among participants with vaccination in the third trimester). (p. 730)
Adverse neonatal outcomes included preterm birth (in 9.4%) and small size for gestational age (in 3.2%); no neonatal deaths were reported. There were 221 pregnancy-related adverse events reported to VAERS, of which the most frequently reported event was spontaneous abortion (46 cases). No congenital anomalies were reported.
Of note, the proportions of adverse pregnancy and neonatal outcomes in the v-safe pregnancy database were similar to those published before the COVID-19 pandemic.”
Exhibit VII: Changes in Table 4
April 2021/2022 NEJM, Shimabukuro:
June 17, 2021, probably after the September 8, 2021, revision:
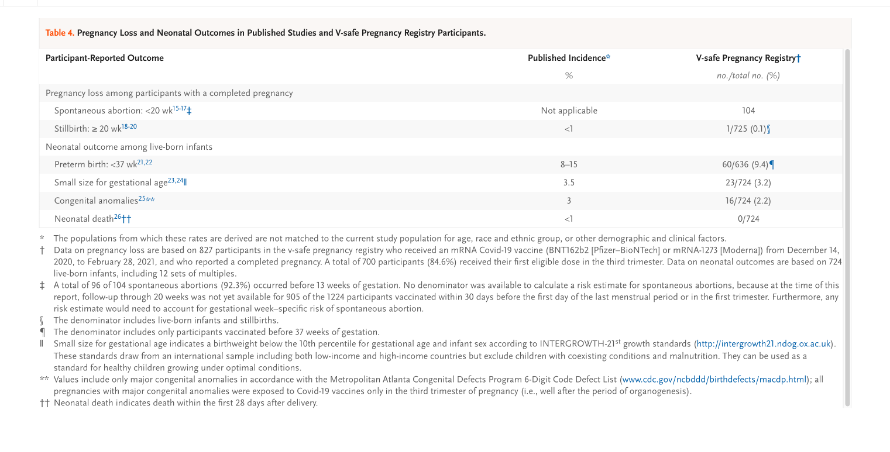
Exhibit VIII: Ongoing clinical trials completed July 15, 2022, with no published results as of September 5, 2022.
This will be a Phase 2/3, randomized, placebo-controlled, observer-blind study evaluating the safety, tolerability, and immunogenicity of 30 µg of BNT162b2 or placebo administered in 2 doses, 21 days apart, in approximately 350 healthy pregnant women 18 years of age or older vaccinated at 24 to 34 weeks’ gestation. Participants will be randomized 1:1 to receive BNT162b2 or placebo (saline).
[https://clinicaltrials.gov/ct2/show/NCT04754594?term=BNT162b2&draw=2&rank=10]
Exhibit IX: Obfuscation
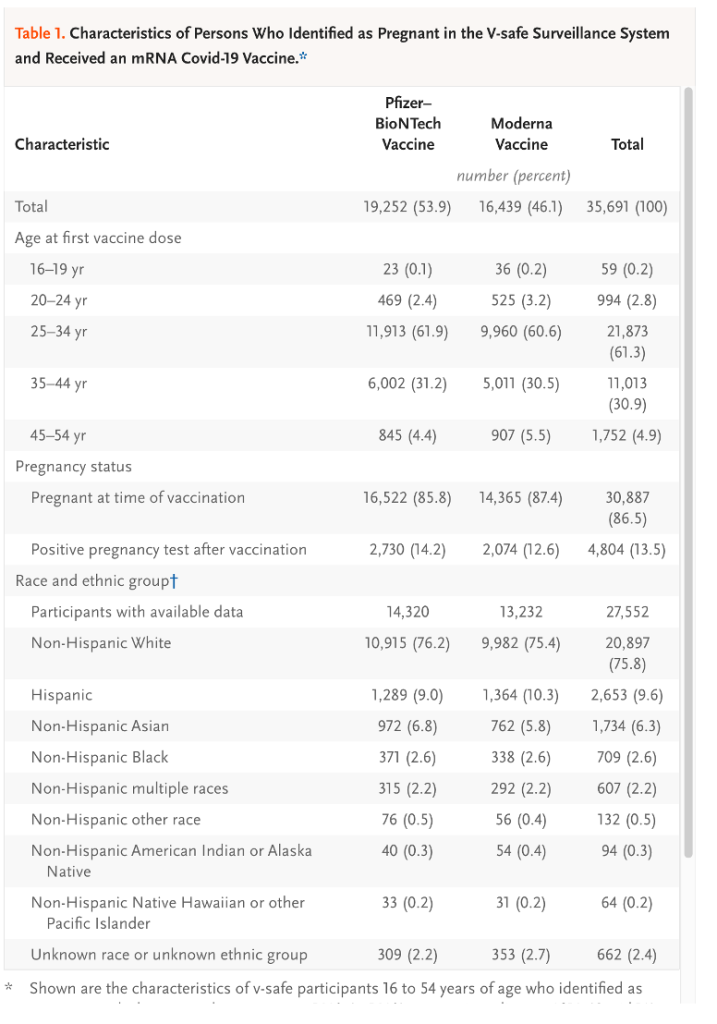
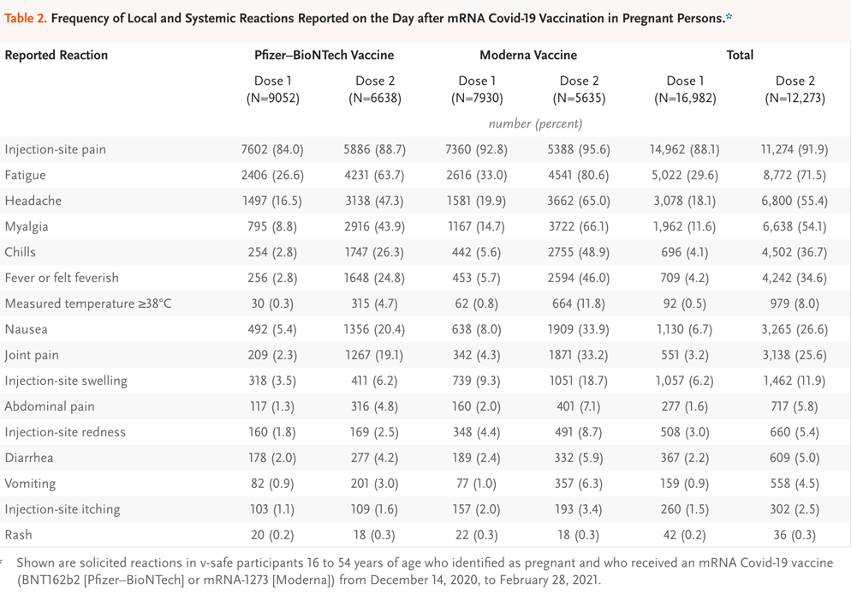
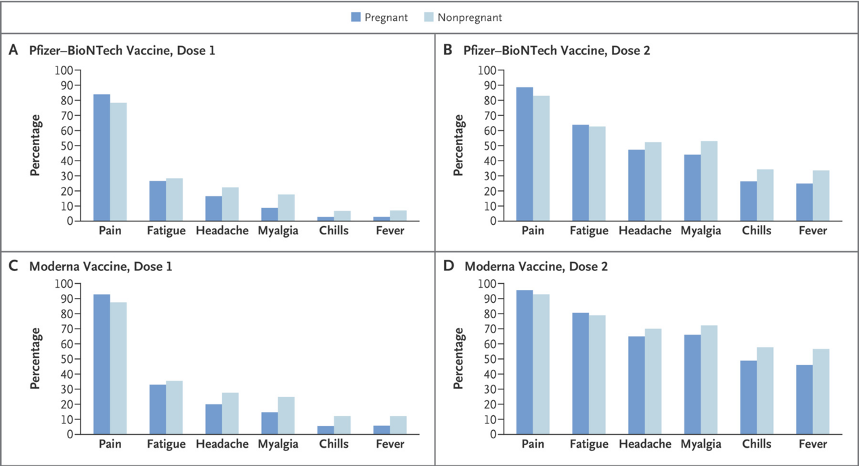
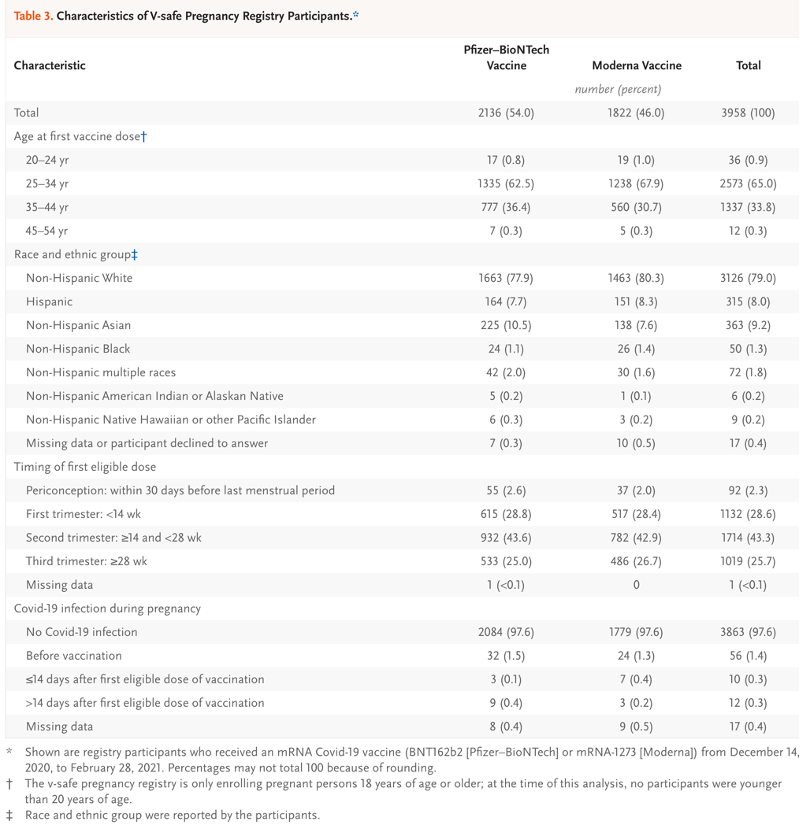
Exhibit X: Authors and Investigators

Adolf Eichmann, May 29, 1962
[https://www.nytimes.com/2016/01/28/world/middleeast/adolf-eichmann-letter-to-israel-president.html?_r=0]
A. Authors and Affiliations of Shimabukuro, et al. NEJM, June 17, 2021.
- Tom T. Shimabukuro, M.D.,
- Shin Y. Kim, M.P.H.,
- Tanya R. Myers, Ph.D.,
- Pedro L. Moro, M.D.,
- Titilope Oduyebo, M.D.,
- Lakshmi Panagiotakopoulos, M.D.,
- Paige L. Marquez, M.S.P.H.,
- Christine K. Olson, M.D.,
- Ruiling Liu, Ph.D.,
- Karen T. Chang, Ph.D.,
- Sascha R. Ellington, Ph.D.,
- Veronica K. Burkel, M.P.H.,
- Ashley N. Smoots, M.P.H.,
- Caitlin J. Green, M.P.H.,
- Charles Licata, Ph.D.,
- Bicheng C. Zhang, M.S.,
- Meghna Alimchandani, M.D.,
- Adamma Mba-Jonas, M.D.,
- Stacey W. Martin, M.S.,
- Julianne M. Gee, M.P.H.,
- Dana M. Meaney-Delman, M.D.,
The authors’ affiliations are as follows:
- Centers for Disease Control and Prevention, Atlanta;
- Immunization Safety Office, Division of Healthcare Quality Promotion (T.T.S., T.R.M., P.L. Moro, L.P., P.L. Marquez, C.K.O., C.L., B.C.Z., J.M.G.)
- Tom T. Shimabukuro, M.D.,
- Tanya R. Myers, Ph.D.,
- Pedro L. Moro, M.D.,
- Paige L. Marquez, M.S.P.H.,
- Christine K. Olson, M.D,
- Charles Licata, Ph.D,
- Bicheng C. Zhang, M.S,
- Julianne M. Gee, M.P.H
- Arboviral Diseases Branch, Division of Vector-Borne Diseases (S.W.M.),
- Stacey W. Martin, M.S.,
- National Center for Emerging and Zoonotic Infectious Diseases, the Division of Birth Defects and Infant Disorders, National Center on Birth Defects and Developmental Disabilities (S.Y.K., V.K.B., C.J.G., D.M.M.-D.),
- Shin Y. Kim, M.P.H.,
- Veronica K. Burkel,
- Caitlin J. Green, M.P.H,
- Dana M. Meaney-Delman, M.D
- Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion (T.O., K.T.C., S.R.E., A.N.S.),
- Titilope Oduyebo, M.D,
- Karen T. Chang, Ph.D,
- Sascha R. Ellington, Ph.D.,
- Ashley N. Smoots, M.P.H.,
- World Trade Center Health Program, National Institute for Occupational Safety and Health (R.L.)
- Ruiling Liu, Ph.D.,
- Epidemic Intelligence Service (K.T.C.) —
- Karen T. Chang, Ph.D.,
- Food and Drug Administration, Silver Spring
7. Division of Epidemiology, Office of Biostatistics and Epidemiology, Center for Biologics Evaluation and Research, Food and Drug Administration, Silver Spring, MD (M.A., A.M.-J.).
- Meghna Alimchandani, M.D,
- Adamma Mba-Jonas, M.D.,
CDC COVID-19 Response v-safe COVID-19 Pregnancy Registry Team
- 1. Amena Abbas, M.P.H.1,
- Dayna Alexander, Ph.D.2 ,
- Katie Arnold, M.D. 3 ;
- Ellen Boundy, Ph.D.4 ;
- Virginia Bowen, M.D. 5 ;
- Kailyn Brooks, M.P.H.6 ,
- Cheryl Broussard, Ph.D.3 ;
- Tara Brown,M.P.H.7 ;
- Gyan Chandra, M.S. 6
- Dena Cherry-Brown, M.P.H.3 ;
- Janet D. Cragan, M.D. 3 ;
- Monica DiRienzo, M.A. 8 ;
- Tuyen Do, B.S.9 ;
- Charise Fox, M.P.H.3 ;
- Suzanne Gilboa, Ph.D.3 ;
- Robbie Gray, A.A.10 ;
- Jennifer Hamborsky, M.P.H.11 ;
- Anne Hause, Ph.D.12 ;
- Jennifer Hegle, M.P.H.13 ;
- Margaret A. Honein, Ph.D.3 ;
- Obehi Ilenikhena, M.P.H.14 ;
- un Ju, M.S.15 ;
- Ashley Judge, M.P.H.3 ;
- Alaya Koneru, M.P.H.11 ;
- Zanie Leroy, M.D.16 ;
- Cynthia Moore, M.D.3 ;
- vAnne Moorman, M.P.H.17 ;
- John Nahabedian, M.S. 3 ;
- Morgan Nail, B.S.18;
- Kimberly Newsome, M.P.H.8 ;
- Keydra Oladapo, M.P.H.19 ;
- Nicole Olgun, Ph.D.20;
- Youngjoo Park, M.P.H.3 ;
- Corinne Marie Parker, Pharm.D.21 ;
- Emily Petersen, M.D. 6 ;
- Kara Polen, M.P.H.3 ;
- Jennita Reefhuis, Ph.D.3 ;
- Penelope Strid, M.P.H.6 ;
- Ayzsa Tannis, M.P.H. 3 ;
- Letha Thomas, Ph.D.13 ;
- Lindsey Torre, M.S.P.H. 13;
- Emmy Tran,Pharm.D.3 ;
- Susanna Trost, M.P.H.6 ;
- Diana Valencia, M.S.3 ;
- Jennifer Linn Wilkers, M.P.H.6 ;
- Lauren Zauche, Ph.D. 3 ;
- Sarah Zohdy, Ph.D.22
- Affiliations: Division of Heart Disease and Stroke Prevention, National Center for Chronic Disease
- Amena Abbas, M.P.H.1,
- Prevention and Health Promotion, CDC;
- Dayna Alexander, Ph.D.2 ,
- Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, CDC;
- Katie Arnold, M.D. 3 ;
- Dena Cherry-Brown, M.P.H.3 ;
- Janet D. Cragan, M.D. 3 ;
- Charise Fox, M.P.H.3
- Suzanne Gilboa, Ph.D.3 ;
- Division of Birth Defects and Infant Disorders, National Center on Birth Defects and Developmental Disabilities, CDC;
- Ellen Boundy, Ph.D.4 ;
- Division of Nutrition, Physical Activity, and Obesity, National Center for Chronic Disease Prevention and Health Promotion, CDC;
- Virginia Bowen, M.D. 5 ;
- Division of Sexually Transmitted Disease Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC;
- Kailyn Brooks, M.P.H.6 ,
- Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, CDC;
- Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, CDC;
- Monica DiRienzo, M.A. 8 ;
- Division of Human Development and Disability, National Center on Birth Defects and Developmental Disabilities, CDC;
- Tuyen Do, B.S.9 ;
- Office of the Director, National Center for Emerging and Zoonotic Infectious Diseases, CDC;
- Robbie Gray, A.A.10 ;
- Office of the Chief Operating Officer, Office of the Director, CDC;
- Jennifer Hamborsky, M.P.H.11 ;
- Immunization Services Division, National Center for Immunization and Respiratory Diseases, CDC;
- Anne Hause, Ph.D.12 ;
- Division of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases, CDC;
- Jennifer Hegle, M.P.H.13 ;
- Division of Global HIV and TB, Center for Global Health, CDC;
- Division of Performance Improvement and Field Services, Center for State, Tribal, Local and Territorial Support, CDC;
- Division of Field Studies and Engineering, National Institute for Occupational Safety and Health, CDC;
- Division of Population Health, National Center for Chronic Disease Prevention and Health Promotion, CDC;
- Division of Viral Hepatitis, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC;
- Division of Laboratory Sciences, National Center for Environmental Health, CDC;
- Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention; 20 Health Effects Laboratory Division, National Institute for Occupational Safety and Health, CDC;
- Division of Preparedness and Emerging Infections, National Center for Emerging and Zoonotic Infectious Diseases, CDC;
- Division of Parasitic Disease Malaria, Center for Global Health, CDC
B. Authors of Zauche, et al., Research Square, August 9, 2021. [https://www.researchsquare.com/article/rs-798175/v1]
- Lauren Head Zauche, Ph.D., M.S.N.1,2,
- Bailey Wallace, M.P.H.,3
- Ashley N. Smoots, M.P.H.,3
- Christine K. Olson, M.D., M.P.H.,4,5
- Titilope Oduyebo, M.D., M.P.H.,3
- Shin Y. Kim, M.P.H.1,
- Emily E. Petersen, M.D.,3,5
- Jun Ju, M.S.,6
- Jennifer Beauregard, Ph.D., M.P.H.,3,5
- Allen J. Wilcox, M.D., Ph.D.,7
- Charles E. Rose, Ph.D.1,
- Dana Meaney-Delman, M.D. M.P.H.,1
- Sascha R. Ellington, Ph.D., M.S.P.H.3
for the CDC v-safe COVID-19 pregnancy registry team
- National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta, GA
- Oak Ridge Institute for Science and Education, U.S. Department of Energy, Oak Ridge, TN, USA
- Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, GA
- Immunization Safety Office, Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention, Atlanta, GA
- United States Public Health Service, Commissioned Corps, Rockville, MD
- National Institute for Occupational Health and Safety, Centers for Disease Control and Prevention, Atlanta, GA
- Epidemiology Branch, National Institute of Environmental Health Sciences, Durham, NC
Investigators
CDC v-safe COVID-19 Pregnancy Registry Team:
- Amena Abbas, M.P.H.1,2,
- Amanda Akosa, M.P.H.3,
- Dayna S. Alexander, Ph.D.4,
- Kathryn E. Arnold, M.D.3,
- Jamal Bankhead, Dr.P.H.5,
- Tabatha Barber, Ph.D.6,
- Amy Blum, M.H.S.A.7,
- Ellen Boundy, Sc.D.8,
- Virginia B. Bowen, PhD.9,
- Kailyn M. Brooks, M.P.H.2,10,
- Cheryl Broussard, Ph.D.3,
- Tara Brown, M.P.H.11,
- Veronica K. Burkel, M.P.H.3,
- Gyan Chandra, M.S.10,
- Karen Chang, Ph.D.12,
- Dena Cherry-Brown, M.P.H.3,
- Monica DiRienzo, M.A.13,
- Tuyen Do, B.S.C.S.14,
- Charise Fox, M.P.H.2,3,
- Julianne M. Gee, M.P.H.15,
- Robbie Gray, A.S.16,
- Caitlin J. Green, M.P.H.3,
- Jennifer Hamborsky, M.P.H.17,
- Anne M. Hause, Ph.D.15,
- Jennifer Hegle, M.P.H.18,
- Obehi Ilenikhena, M.P.H.19,
- Jessly Joy, M.P.H.20,
- Ashley Judge, M.P.H.2,3,
- Alaya Koneru, M.P.H.17,
- Zanie Leroy, M.D.21,
- Birook Mekonnen, M.P.H.2, 13,
- Anne Moorman, M.P.H.22,
- Pedro L. Moro, M.D.15,
- John F. Nahabedian III, M.S.3,
- Morgan Nail, B.S.2, 23,
- Andrea Neiman, Ph.D.1,
- Kimberly Newsome, M.P.H.13,
- Suzanne Newton, M.P.H. 3,
- Keydra Phillips Oladapo, Ph.D.5,
- Nicole S. Olgun, Ph.D.6,
- Pamela Pagano,
- P.H.10, Lakshmi Panagiotakopoulos, M.D.22,
- Youngjoo Park, M.P.H.2,3,
- Corrine Parker, Pharm.D.12,
- Kara Polen, M.P.H.3,
- Emmitt Rathore, B.S.3,
- Tom T. Shimabukuro, M.D.15,
- Penelope Strid, M.P.H.10,
- Jennifer M. Swanson, M.P.H.18,
- Ayzsa F. Tannis, M.P.H.2,3,
- Naomi K. Tepper, M.D.3,
- Letha Thomas, Ph.D.18,
- Lindsey A. Torre, M.S.P.H18,
- Emmy L. Tran, Pharm.D.3,
- Susanna L. Trost, M.P.H.2,10,
- Diana Valencia, M.S.3,
- Jennifer L. Wilkers, M.P.H.2, 10,
- Luke Yip, M.D.24, and
- Sarah Zohdy, Ph.D25
- Division of Heart Disease and Stroke Prevention, National Center for Chronic Disease Prevention and Health Promotion, CDC;
- Oak Ridge Institute for Science and Education;
- Division of Birth Defects and Infant Disorders, National Center on Birth Defects and Developmental Disabilities, CDC;
- Division of Diabetes and Translation, National Center for Chronic Disease Prevention and Health Promotion, CDC;
- Division of HIV/AIDS Prevention National Center for HIV/AIDS, Viral Hepatitis, STD, and TB prevention;
- Health Effects Laboratory Division, National Institute for Occupational Safety and Health, CDC;
- Division of Health Care Statistics, National Center for Health Statistics, CDC;
- Division of Nutrition, Physical Activity, and Obesity, National Center for Chronic Disease Prevention and Health Promotion, CDC;
- Division of Sexually Transmitted Disease Prevention, National Center for HIV/AIDS, Vital Hepatitis, STD and TB Prevention, CDC;
- Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion;
- Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, CDC;
- Division of Preparedness and Emerging Infections, National Center for Emerging and Zoonotic Infectious Diseases, CDC;
- Division of Human Development and Disability, National Center on Birth Defects and Developmental Disabilities, CDC;
- Office of the Director, National Center for Emerging and Zoonotic Infectious Diseases, CDC;
- Division of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases, CDC;
- Office of the Chief Operating Officer, Office of the Director, CDC;
- Immunization Services Division, National Center for Immunization and Respiratory Diseases, CDC;
- Division of Global HIV and TB, Center for Global Health, CDC;
- Division of Performance Improvement and Field Services, Center for State, Tribal, Local and Territorial Support, CDC;
- Division of Analysis and Epidemiology, National Center for Health Statistics, CDC;
- Division of Population Health, National Center for Chronic Disease Prevention and Health Promotion, CDC;
- Division of Viral Hepatitis, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC;
- Division of Laboratory Sciences, National Center for Environmental Health, CDC;
- National Center for Environmental Health/Agency for Toxic Substances and Disease Registry, Office of Emergency Management, CDC;
- Division of Parasitic Disease Malaria, Center for Global Health, CDC.




I am paid 114 dollars per hour to perform certain internet services online. I had no idea it was feasible, but my closest friend joined and earned $27 thousand in just five weeks by doing sac-15 this simple task. Visit for greater information about visiting this page. Anyone may obtain this right away and begin making money online.
by following the directions on this website………………. https://smartpay21.pages.dev
Question regarding those who are of reproductive age who have had the first two shots specifically, no boosters and then become pregnant months or even a year/years later. Does this same dangerous situation apply to their unborn children, ie; spontaneous miscarriages, breastmilk being toxic, etc…..
I have 4 daughters, 2 of whom because of work got the initial shots, now regretting and don’t want anymore. They are in fear that if they were to get pregnant now, they and their unborn children could suffer these horrific consequences.
What, if anything is known about the dangers to future pregnancies after having the shots?
Of course as their mother I am heartbroken for them and pray they will be able to have successful pregnancies and healthy children.
God in heaven help us!