Letter to the FDA: Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT), a New and Dangerous Syndrome from Certain COVID Vaccines – Do We Have Them All?

Introduction
- WHAT IS “VITT”?
Vaccine-induced Immune Thrombotic Thrombocytopenia (VITT) is “a newly described syndrome” “characterized by new onset thrombocytopenia [i.e., low blood platelet count] and acute venous or arterial thrombosis [i.e., blood clot], including at unusual sites such as the cerebral venous sinus, with onset of symptoms approximately one to two weeks following receipt of the [certain COVID-19] vaccine[s].” Other names for VITT are: Thrombosis with Thrombocytopenia Syndrome (TTS) or Vaccine-induced Prothrombotic Immune Thrombocytopenia (VIPIT).[https://www.hematology.org/covid-19/vaccine-induced-immune-thrombotic-thrombocytopenia, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8063912/, https://www.fda.gov/media/158318/download, and https://www.uptodate.com/contents/covid-19-vaccine-induced-immune-thrombotic-thrombocytopenia-vitt]
- DIDN’T WE HEARD ABOUT “VITT” EARLIER THIS YEAR?
Yes, on May 5, 2022, the U.S. Food and Drug Administration (FDA) limited the Emergency Use Authorization (EUA) of Janssen’s COVID-19 vaccine based on the Vaccine Adverse Event Reporting System (VAERS) data showing 54 cases of confirmed TTS [VITT]. “[C]lustering in time after vaccination” was determined. The vaccine could then only be used when “…other FDA-authorized or approved COVID-19 vaccines are not accessible or clinically appropriate.” This “limit” continues to this day; the vaccine has never made it to full approval. Regarding the mRNA vaccines, no such limit was imposed: “…the reporting rate for TTS after mRNA COVID-19 vaccination… found here falls below that estimate of background incidence before COVID-19 vaccines were available.”
[https://www.fda.gov/media/158318/download and https://www.acpjournals.org/doi/10.7326/M21-4502]
A special note of concern: While one of the diagnostic criterion for VITT is, “Any venous or arterial thrombosis (often cerebral or abdominal),” and while arterial presentations for VITT may include ischemic stroke and myocardial infarction, the CDC’s “…working case definition for TTS [VITT] following covid-19 vaccine” excluded “[r]eports where only thrombosis is ischemic stroke or myocardial infarction.”
[https://www.hematology.org/covid-19/vaccine-induced-immune-thrombotic-thrombocytopenia, https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-12-16/02-COVID-See-508.pdf, and https://www.uptodate.com/contents/covid-19-vaccine-induced-immune-thrombotic-thrombocytopenia-vitt]
- IF VITT IS A “NEWLY DESCRIBED CONDITION” ATTRIBUTED TO A TYPE OF COVID-19 VACCINE, CAN “BACKGROUND INCIDENCE BEFORE COVID-19 VACCINES WERE AVAILABLE” BE USED AS A SIGNAL DETECTION TOOL?
While there may be clinical conditions that present similarly to VITT (such as cerebral venous sinus thrombosis (CVST) or spontaneous heparin-induced thrombocytopenia (spontaneous HIT)), VITT did not exist in the pre-COVID-19 vaccine era, so it is not clear how assessment of current VITT reporting rates against the background incidence of a separate, better-characterized clinical condition would be valid. Additionally, true incidence cannot be derived from a passive surveillance system such as VAERS.
[https://www.acpjournals.org/doi/10.7326/M21-4502, https://n.neurology.org/content/99/15/672.1, and https://globalforum.diaglobal.org/issue/december-2021/monitoring-safety-signals-for-vaccines-current-strategies-and-future-directions/]
- IS THE JANSSEN VACCINE THE ONLY COVID-19 VACCINE OF CONCERN FOR VITT?
The May 2022 limit of the Janssen COVID-19 vaccine EUA was, in part, based on “…the clustering in time [of 54 confirmed VITT cases] after vaccination.” Today, the VITT cases reported to VAERS following the mRNA vaccines total 60 for Pfizer and 26 for Moderna, respectively, and also appear to be “…clustering in time…” “…with onset of symptoms approximately one to two weeks following receipt of the vaccine…” in approximately half the cases. In the case of the “newly described syndrome,” VITT, where there can be no “…expected number of occurrences estimated from background incidence,” the number of cases “…observed in temporal relationship to vaccination,” is of paramount importance. As awareness of VITT is still evolving among clinicians, reporting rates for VITT may be unreliable due to “[p]ossible underdiagnosis of CVST and TTS.” In my letter, cases of CVST reported following mRNA vaccination (611 for Pfizer and 169 for Moderna) are also included as a possible indicator for VITT (TTS).
[https://globalforum.diaglobal.org/issue/december-2021/monitoring-safety-signals-for-vaccines-current-strategies-and-future-directions/, https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-12-16/02-COVID-See-508.pdf, and https://www.fda.gov/media/158318/download]
Dr. Taccetta’s Letter to Dr. Peter Marks, Director of the Center for Biologics Evaluation and Research (CBER) at the Food and Drug Administration (FDA)
October 14, 2022
Dear Dr. Marks,
I appreciate the FDA’s timely response back to me: “FDA does not consider TTS a safety signal following vaccination with the Pfizer-BioNTech or Moderna COVID-19 vaccines.”*
*Thrombosis with Thrombocytopenia Syndrome (TTS) is also termed Vaccine-induced Prothrombotic Immune Thrombocytopenia (VIPIT) or Vaccine-induced Immune Thrombotic Thrombocytopenia (VITT).
[…]
My concerns with comparing rates of TTS (or cerebral venous sinus thrombosis (CVST) as an indicator for TTS) with “background rates” of other conditions (as suggested by others) are multiple: https://www.acpjournals.org/doi/10.7326/M21-4502
- TTS is its own novel, recently described condition following novel covid-19 vaccines (first described late February 2021): […] Our evolving understanding of its pathogenesis precludes comparison to rates of a better- characterized condition, such as spontaneous heparin-induced thrombocytopenia (HIT) syndrome. https://www.ncbi.nlm.nih.gov/books/NBK570605/
- TTS is not yet fully understood by all medical professionals. It may not be appropriately reported. Additionally, thrombocytopenia may not appear early on in TTS, leading to misdiagnosis. https://www.hematology.org/covid-19/vaccine-induced-immune-thrombotic-thrombocytopenia
- Regardless of the reporting rates of TTS/CVST following the mRNA vaccines, there is clearly a strong temporal association: in approximately half the TTS or CVST reports observed in VAERS, there is ‘clustering’ within 14 days of vaccination (the American Society of Hematology syndrome criterion is actually up to 42 days post-vaccination). https://www.hematology.org/covid-19/vaccine-induced-immune-thrombotic-thrombocytopenia
- CVST is rare. TTS is rare. Spontaneous HIT is rare. “Only in rare cases does cerebral venous sinus thrombosis or thrombi in abdominal vessels develop in patients with heparin-induced thrombocytopenia. This suggests that our understanding of the pathogenesis of VITT is incomplete.” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8063912/
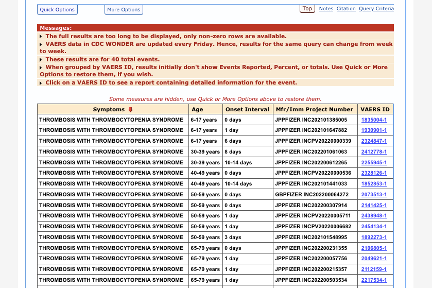
As of 07 OCT 2022:
There are 611 total CVST events reported for Pfizer’s vaccine:
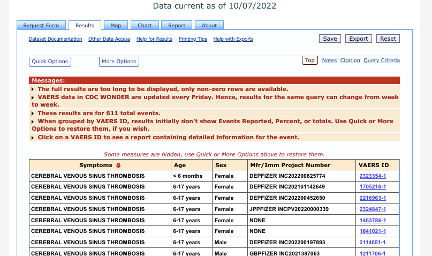
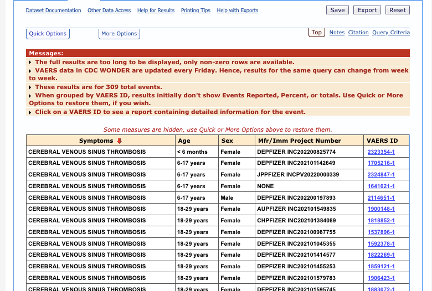
309/611 had an onset within 14 days post-vaccination:
There are 169 total CVST events reported for Moderna’s vaccine:
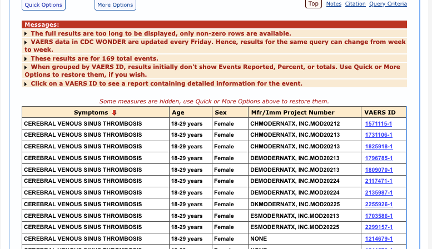
73/169 had an onset within 14 days post-vaccination:
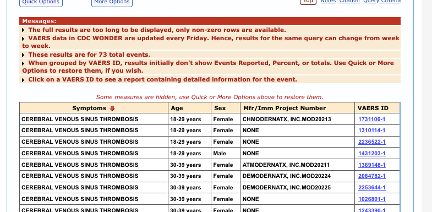
Out of 26 total, 14 TTS cases were reported in association with Moderna’s vaccine with onset within 14 days:
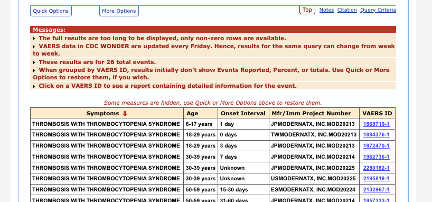
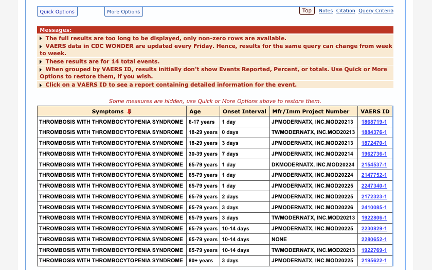
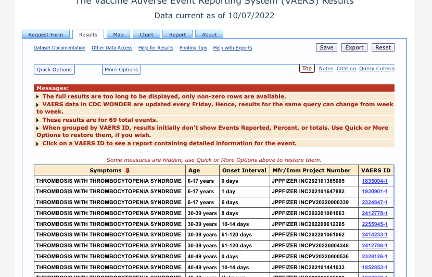
Out of 69 total, 40 TTS cases were reported in association with Pfizer’s vaccine with onset within 14 days:
One death due to this elusive syndrome is one too many. At this point, a temporary pause of the mRNA vaccines for thorough evaluation of the available safety data is justified.
I await your response to this critical concern.
Sincerely,
Carol Taccetta, MD, FCAP




I just worked part-time from my apartment for 5 weeks, but I made $30,030. I lost my former business and was soon worn out. Thank goodness, “ed40] I found this employment online and I was able to start working from home right away. This top career is achievable by everyone, and it will improve their online revenue by:.
EXTRA DETAILS HERE:>>> http://dosmartwork24.blogspot.com