Report 10: Even Big Pharma CEOs Recognized That Not Everyone Could be Vaccinated – So Why the Mandates?
Recently, Project Veritas revealed that the CEO of AstraZeneca, Pascal Soriot, told his company in a Zoom call in Dec 2020 that not everyone could be vaccinated; Soriot identified the immune-compromised and people with multiple sclerosis as examples if those who should not be vaccinated with mRNA vaccines. He raised this issue in the context of explaining that the company AstraZeneca had a great opportunity in the marketplace — to make antibody treatments for those vulnerable populations, treatments, that is, which could give protection to those who should not be vaccinated.
Project Veritas broke the story on April 19, 2022, where Soriot admits that immunocompromised populations should not consider the AstraZeneca vaccine safe.
YouTube also has this incriminating video.
Soriot’s comments were contradictory to remarks about the safety of the vaccine for immunocompromised people made by the World Health Organization (WHO) at the time. More recently, on March 16, 2022, a Health Advisory from the WHO restated the assertion that the vaccine was SAFE for immunocompromised individuals. (Time marker: 39 mins) Those statements appear to give false assurance.
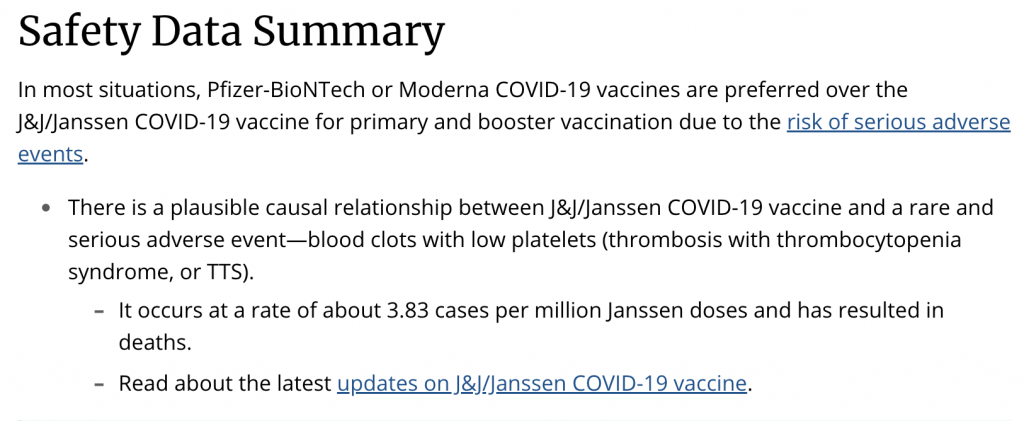
There have been serious problems with the AstraZeneca vaccine even for the general population. AstraZeneca is the maker of one of the main COVID vaccines used in Europe, which along with Johnson and Johnson’s (Janssen vaccine) has been plagued with reports of the vaccines’ causing small vessel blood clots:

In admitting the fact that vaccine-induced immunity is not viable for immunocompromised patients, AZ saw the commercial opportunity to develop and manufacture monoclonal antibodies against the S (SPIKE) protein.
This is the important argument that they make, in stark contrast to the CDC and FDA pronouncements in the USA where vaccine mandates were National Policy, that you cannot produce antibodies to a vaccine if you are immunocompromised and need to have a different source of antibodies.
Why should this matter in the US?
AstraZeneca (AZ), like Johnson and Johnson, used a conventional approach of a modified viral vector (rather than using mRNA) for producing immunity. AZ recognized the issues this would create with patients whose natural immunity was depressed due to illness or to chemotherapy drugs (a state known as being ‘immunocompromised’).
So why weren’t Monoclonal antibodies the first line of attack against COVID?
Steps were taken by several States, who targeted their vulnerable populations with protective efforts (such as closing visits to care homes in the early days), and purchased monoclonal antibodies to use in the fight against COVID. Vaccines were not available until late November 2021.
Patients with a compromised immune system could have their immunity provided by externally administered antibodies.
Antibodies from patients who had recovered from COVID, known as Convalescent Plasma was first approved by the FDA in August 2020.
In November, 2021, the FDA approved the first two monoclonal antibody treatments manufactured by Regeneron Pharmaceutical Inc (Casirivimab and Imdevimab)
Subsequently monoclonal antibodies became one of the important mainstays of treatment in a number of US States, where the priority was to protect the vulnerable population, rather than to make use of a ‘one size fits all’ vaccine treatment.
So why mandate a vaccination for 100% of the population
if vaccination is NOT effective for immunocompromised patients?
If the CEOs of Vaccine Manufacturers can recognize the lack of effectiveness in part of the population, why do the CDC/FDA as well as W.H.O. continue to advocate for additional boosters for these patients? In view of the serious side effects of the mRNA vaccines already known, why are they still being mandated?
The only conclusion that I can come to is that vaccine mandates are both unwise and downright wrong.



