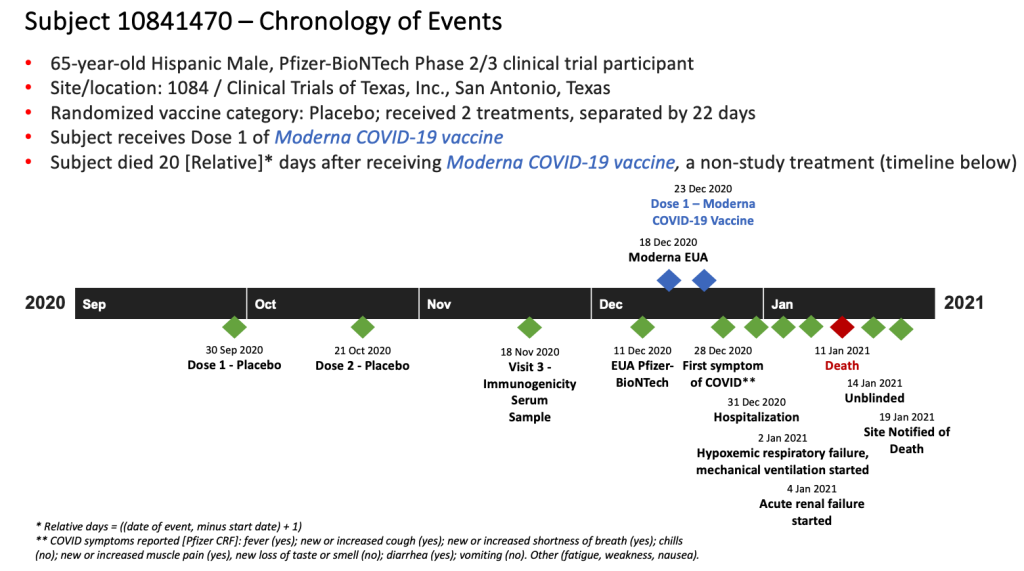
Report 82: Pfizer Clinical Trial Subject Dies Soon After Receiving One Dose of Moderna COVID Vaccine. Forty-Two Days Post-Mortem, Pfizer Staff Seem Unaware He Is Dead.
A protocol keeps a clinical trial accurate and rigorous. The 361-page Case Report Form (CRF) for Pfizer clinical trial subject 10841470 shows that Pfizer allowed significant deviations from its protocol, such as not discontinuing the subject from the trial when he first took a different brand of COVID vaccine and then later received monoclonal antibodies, both trial-prohibited medications. The subject also previously participated in a study involving an intervention containing lipid nanoparticles, which should have excluded him from entering in the trial in the first place. Not removing the subject from the trial based on these deviations and ignored exclusion criterion contaminates the trial and its results, according to standard scientific norms, while also leaving one to wonder what other exclusion criteria were ignored and protocol deviations occurred in the Pfizer trial.
Case Report Form (CRF) for Pfizer clinical trial subject 10841470 offers readers an inside view of a subject’s (i.e., patient’s) experiences in Pfizer’s COVID-19 vaccine clinical trial, both when entering and then going through the clinical trial process. Pfizer captures each subject’s data from its first encounter with a patient through the end of his or her participation in the trial. What this CRF reveals is shocking:
- A clearly unhealthy person was not excluded from the trial, which is at the investigator’s discretion.
- Trial participants can easily receive non-Pfizer COVID vaccines and choose to report them…or not.
- Subject chose to get Moderna vaccine despite not knowing if he had received two doses of the Pfizer vaccine or the placebo.
- Based on his knowledge at the time, he could have been receiving a third dose of an mRNA/LNP vaccine in under three months.
- Onset of COVID only five days after receiving one dose of Moderna.
- Hospitalization with severe COVID, requiring monoclonal antibodies, only eight days after one dose of Moderna.
- Mechanical ventilation two days after entering hospital.
- Death 20 days after Moderna Dose 1.
- Documentation in the CRF does not support multi-organ dysfunction syndrome as primary cause of death or earlier diagnosis of acute renal failure.
- Subject is unblinded – i.e., documented has being a Pfizer placebo patient, not a vaccine patient – three days post-mortem.
- Pfizer personnel change data in the CRF after his death, including his September 30, 2020, answers to the inclusion/exclusion questions.
- Pfizer gave him Dose 1 and Dose 2 without having documented whether or not he was HIV positive or negative.
- Pfizer documents his reason for discontinuation from the trial as being “death,” when it should have been documented as protocol deviations.
- Is it fraud to misrepresent the real reason for discontinuation?
- Forty-two days after subject’s death, Pfizer personnel still seem unaware of his death, despite it being documented in the CRF on January 19, 2021.
- At that time, on February 22, 2021, personnel write that he was unblinded to be assessed for receiving Dose 3 (the Pfizer vaccine).

What Are Case Report Forms (CRFs)?
Clinical trials, such as Pfizer’s mRNA COVID-19 vaccine trial, use Case Report Forms (CRFs) and electronic Case Report Forms (eCRFs) to collect patient, or subject, data. “A case report form (CRF) is designed to collect the patient data in a clinical trial; its development represents a significant part of the clinical trial and can affect study success. Site personnel capture the subject’s data on the CRF, which is collected during their participation in a clinical trial. The International Conference on Harmonization Guidelines for Good Clinical Practice define the CRF as: A printed, optical or electronic document designed to record all of the protocol – required information to be reported to the sponsor on each trial subject.” (Bellary, Shantala, et al. “Basics of Case Report Form Designing in Clinical Research.” Perspectives in Clinical Research, U.S. National Library of Medicine, Oct. 2014, www.ncbi.nlm.nih.gov/pmc/articles/PMC4170533/.)
Subject 10841470 Enters the Pfizer mRNA COVID-19 Vaccine Clinical Trial (Visit 1) – September 30, 2020
Upon entering Pfizer’s trial, participants’ medical histories and vitals are collected. Pfizer trial Subject 10841470 was a 65-year-old, White Hispanic/Latino male associated with Pfizer site number 1084, Clinical Trials of Texas. [p. 3.] With a Body Mass Index (BMI) of 41.2 and weighing 130.4 kg (287.5 lbs.) at a height of 177.8 cm (5 feet, 10 inches), he was clinically obese. Additionally, his medical history included pulmonary fibrosis (start 2014, ongoing), hypertension (start 2010, ongoing); GERD (gastroesophageal reflux disease) (start 2010, ongoing), insomnia (start 2010, ongoing), and hyperlipidemia (start 2010, ongoing). [pp. 11 and 13.]
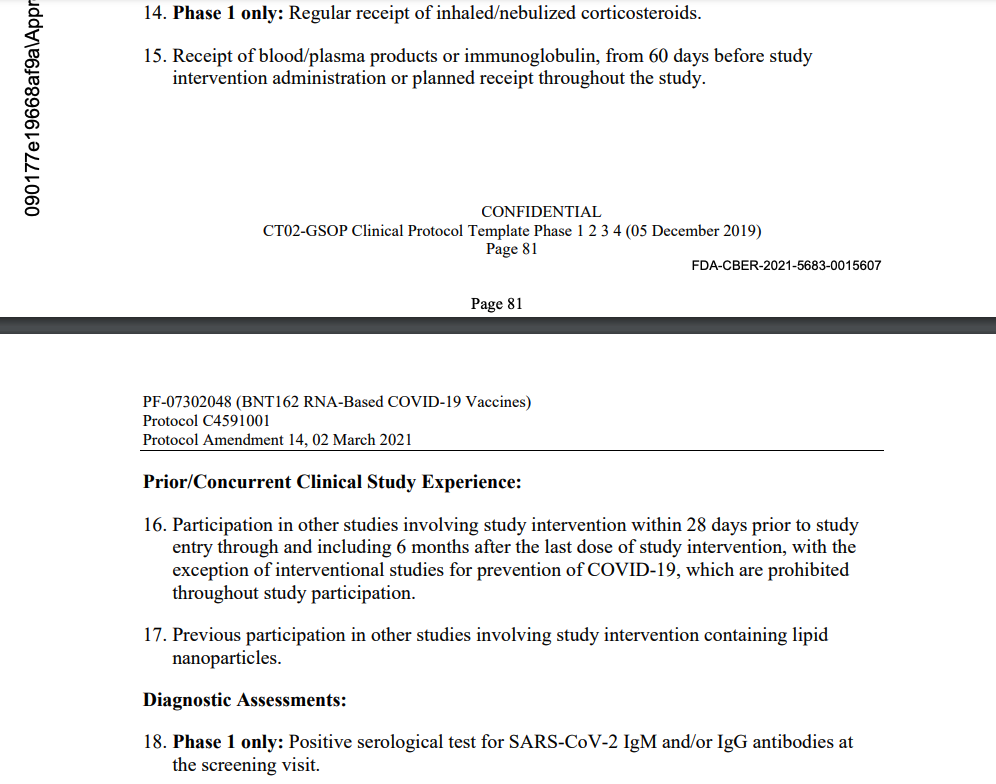
Despite his apparent poor health and on-going pulmonary fibrosis disease, this subject was not deemed unhealthy enough to meet Pfizer’s Phase 2/3 Exclusion Criteria outlined in its protocol and shown below for “A PHASE 1/2/3, PLACEBO-CONTROLLED, RANDOMIZED, OBSERVER-BLIND, DOSE-FINDING STUDY TO EVALUATE THE SAFETY, TOLERABILITY, IMMUNOGENICITY, AND EFFICACY OF SARS-COV-2 RNA VACCINE CANDIDATES AGAINST COVID-19 IN HEALTHY INDIVIDUALS,” as seen below. [pp. 80-82] Those criteria noted as being “Phase 1 only” do not apply to Subject 10841470. (Note: the protocol link goes to “Protocol Amendment 14” so that the reader may see all six of the previous protocol amendments covering the span of time for this patient’s involvement with the trial, September 30, 2020, through January 11, 2021. [pp. 4-7])


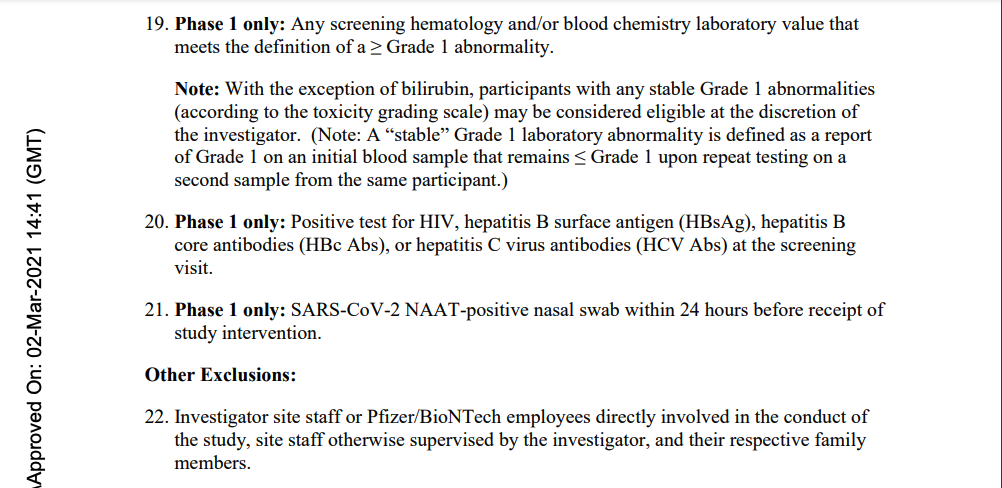
It appears that Subject 10841470 was deemed “healthy” based on his medical history, a possible physical examination, and the clinical judgment of the investigator” and, thus, met Pfizer’s Phase 2/3 Inclusion Criteria:


Subject 10841470 was initially screened, randomized into the placebo cohort of the study, and received Dose 1 of the placebo on September 30, 2020 (Visit 1). At the time of receiving Dose 1, he was observed post-injection for the length of time required per protocol, had his temperature checked, and had study serum samples and nasal swab samples collected. No reactions were noted.
Subject’s Second Pfizer Clinical Trial Visit – October 21, 2020
On October 21, 2020 (Visit 2), Subject 10841470 received Dose 2 (again placebo) and was then observed post-injection for the length of time required per protocol, had his temperature checked, and had nasal swabs collected. No reactions were noted.
Subject’s Third Pfizer Clinical Trial Visit – November 18, 2020
On November 18, 2020 (Visit 3), almost one month after Dose 2, an immunogenicity serum sample was collected, which completed the subject’s vaccination phase.
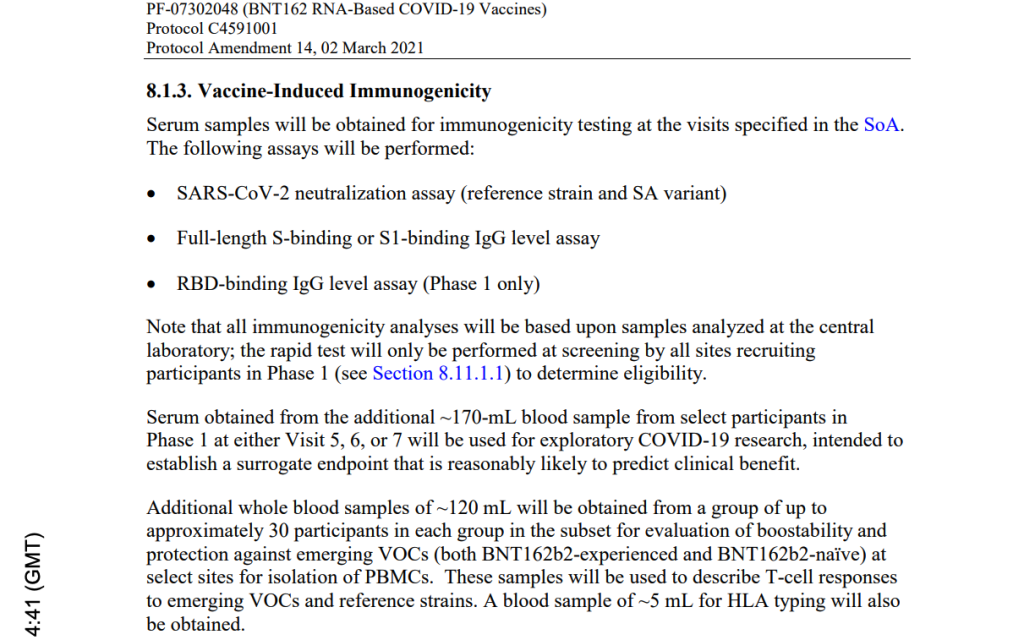
Subject Receives Dose 1 of the MODERNA mRNA COVID-19 Vaccine – December 23, 2020
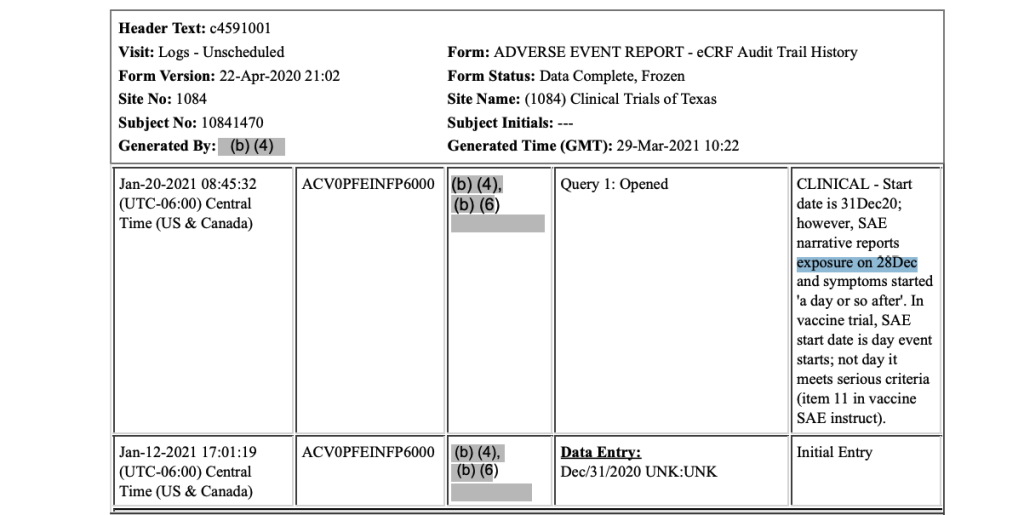
Unbeknownst to the Pfizer study site, Subject 10841470 received his first dose of the Moderna mRNA COVID-19 vaccine, a concomitant medication, on December 23, 2020. This is protocol deviation that should have excluded him from further participation in Pfizer’s trial. [https://phmpt.org/wp-content/uploads/2023/08/125742_S1_M5_CRF_c4591001-1084-10841470.pdf, pp. 87-88.]
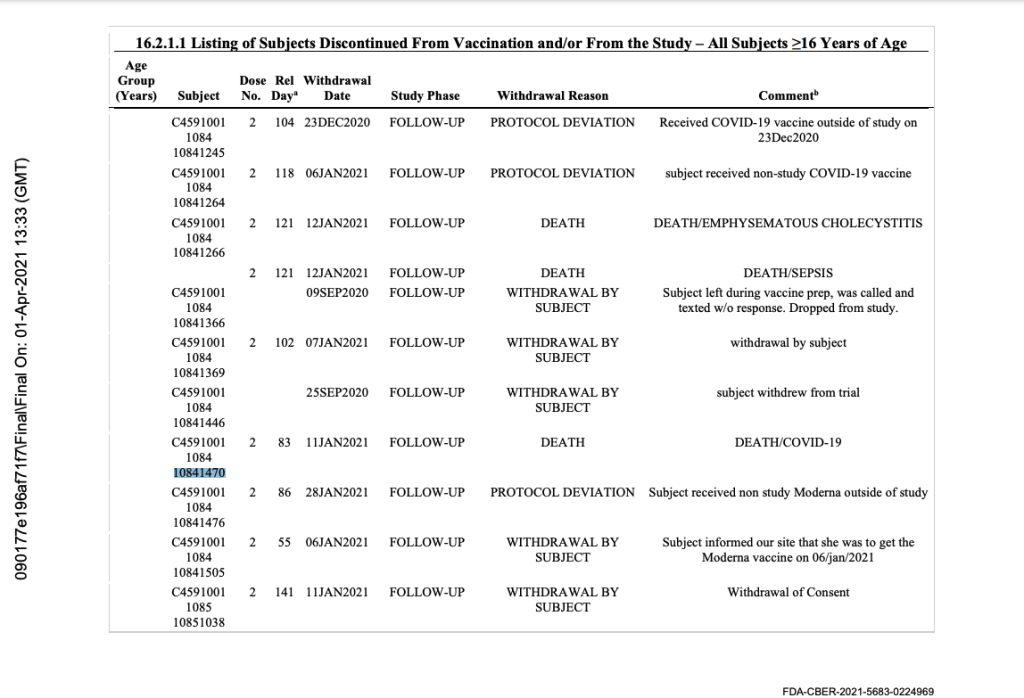
However, in Pfizer’s 125742_S1_M5_5351_c4591001-interim-mth6-discontinued-patients.pdf document, Subject 10841470 is noted as being discontinued from the trial due to death, not a protocol deviation, even though the protocol deviation occurred prior to his death:
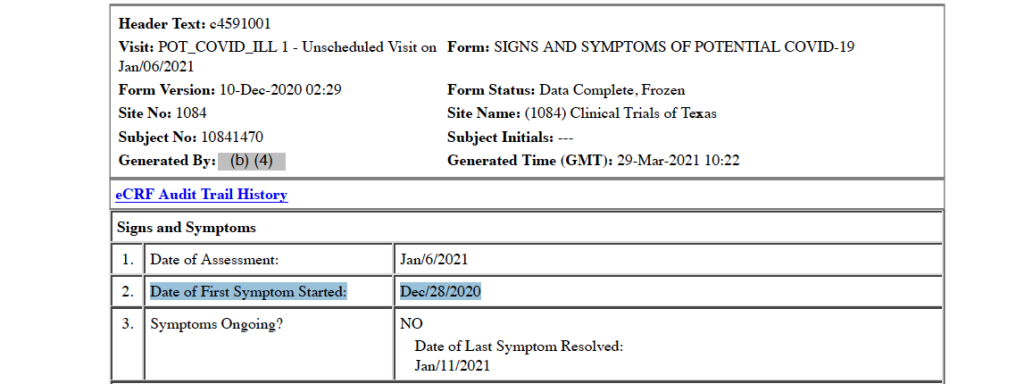
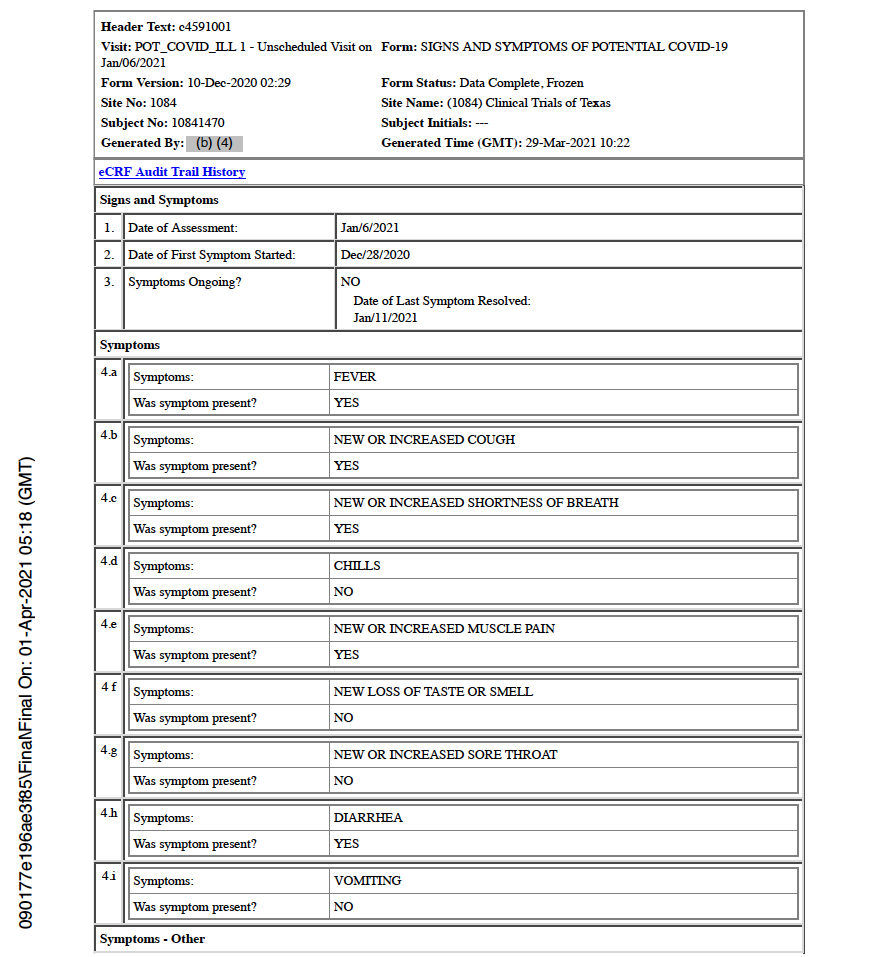
Subject Experiences His First COVID-19 Symptom – December 28, 2020
Just five days later, the trial participant experienced his first COVID-19 symptom. Though the main body of the CRF documents this date as the occurrence of his first COVID symptom, the CRF later states that the December 28 date represents the subject’s initial COVID-19 “exposure” and includes an unofficial, allegedly asymptomatic positive outpatient COVID-19 test. [pp. 32, 358-359]
Emergency Hospitalization of Subject – December 31, 2020
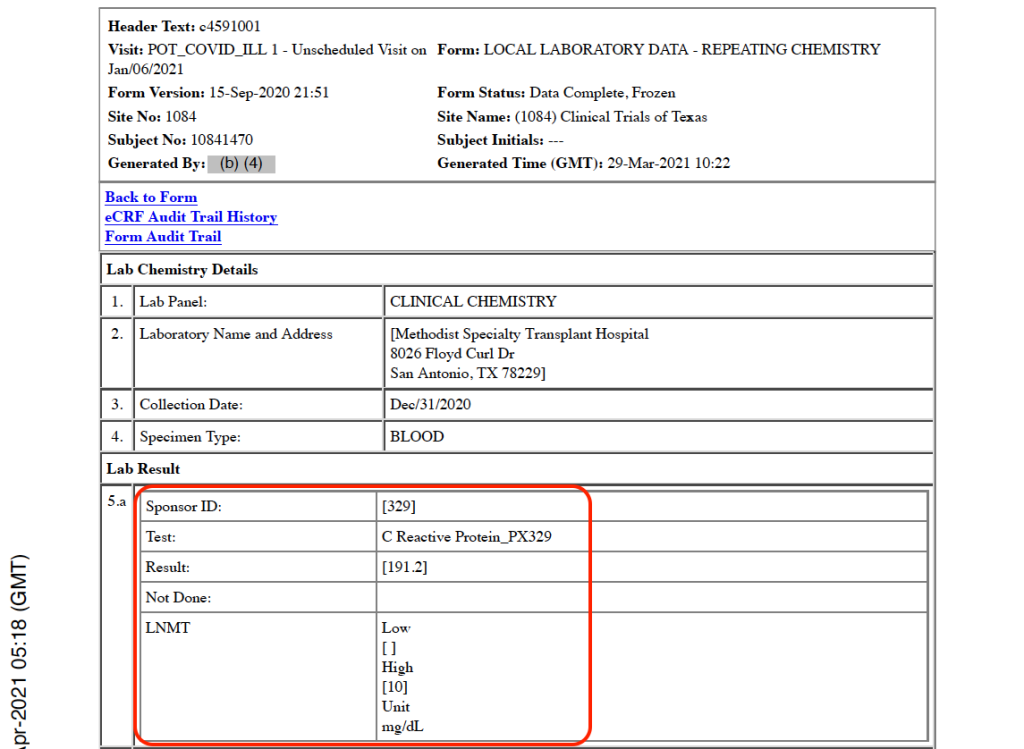
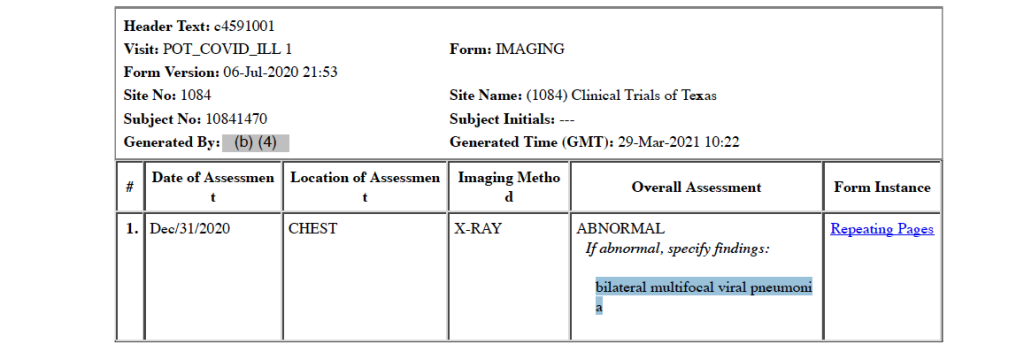
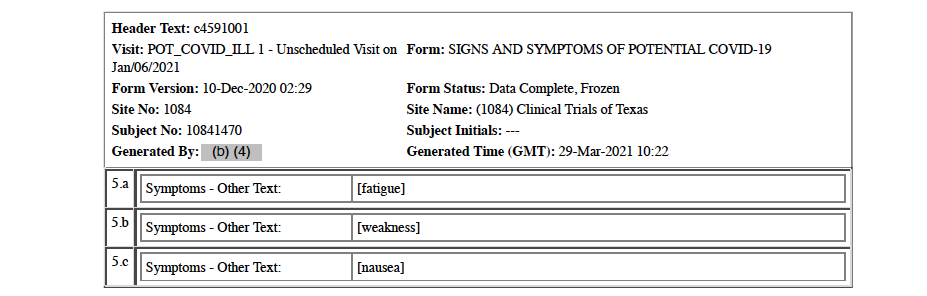
The first positive, official COVID-19 test is recorded in the CRF as occurring on December 31, 2020, when the subject presents to the Methodist Specialty Transplant Hospital (San Antonio, Texas) emergency room with COVID-19 infection requiring intensive care hospitalization. His symptoms include fever, new or increasing cough, new or increasing shortness of breath, new or increasing muscle pain, diarrhea, fatigue, weakness, and nausea. [pp. 32-33] His C-Reactive Protein level, an inflammation marker, was severely elevated at 191.2 mg/dL [p. 47], and his chest X-ray was abnormal showing bilateral multifocal viral pneumonia [p. 72]. Other clinical chemistry and hematology results on this date were: BUN (renal function marker) 15 mg/dL (normal 9-20); serum creatinine (renal function marker) 1.10 mg/dL (normal 0.4-1.2); and platelets 176 units/microliter (normal 150-400). It is noteworthy leukocytes and lymphocytes, both white blood cells, results were left blank.
The hospital gave him monoclonal antibodies, another Pfizer trial-prohibited concomitant medication and, thus, another protocol deviation.
The subject’s vital signs noted for December 31,2020, the date of hospital admission, were: blood pressure 171/94, respiration of 18 breaths per minute, oxygen saturation of 88.0 (low), and heart rate of 84 beats per minute.
Subject Is Mechanically Ventilated and Then Suffers Acute Renal Failure – January 2, 2021, through January 4, 2021
Subject 10841470 treated at bedside for acute hypoxemic respiratory failure, intubated (mechanical ventilation), and sedated on January 2, 2021.
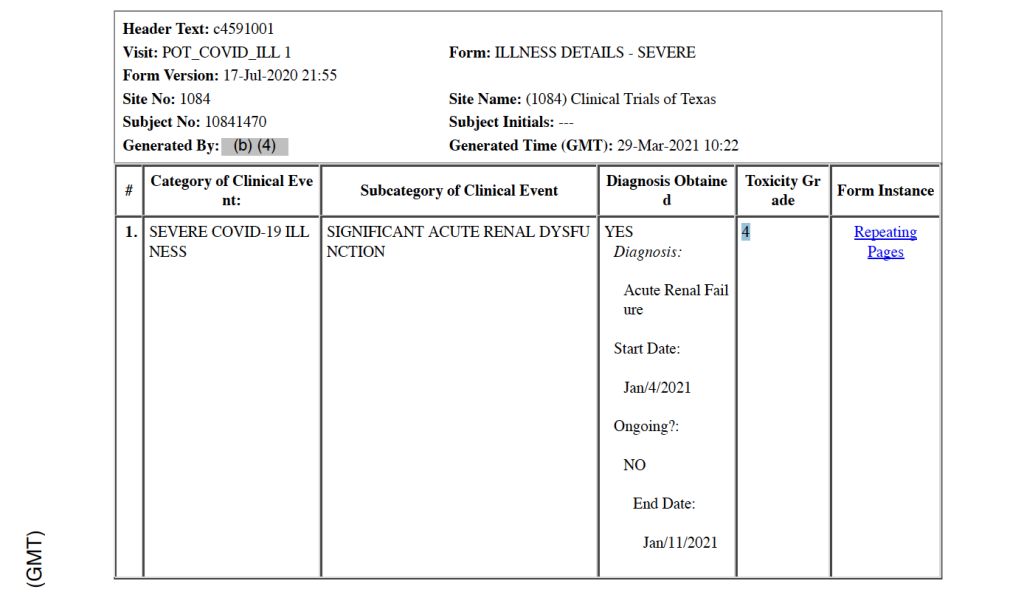
He then goes into acute renal failure on January 4, 2021, which is noted with toxicity Grade 4 and as a subcategory of the Severe COVID-19 Illness clinical event category. [pp. 44-45]
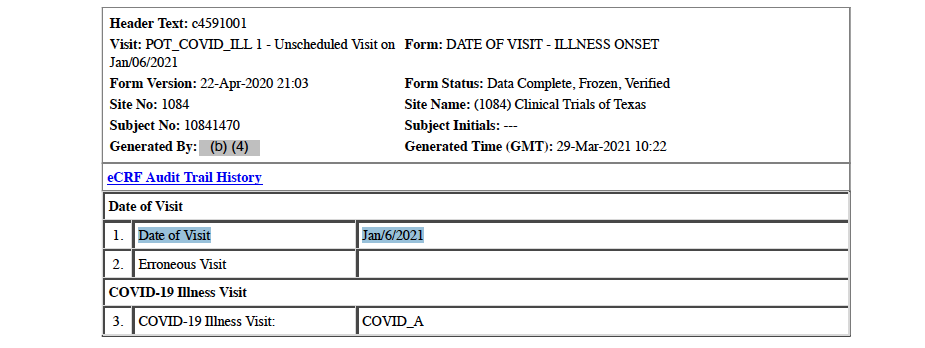
Potential COVID-19 Illness Visit Recorded for Subject – January 6, 2021
Strangely, a January 6, 2021, “Potential COVID-19 Illness Visit” with a health assessment is documented in the CRF. [pp. 31-33] The subject was in the hospital, on a ventilator, and in acute renal failure on that date and would have been unable to go into the study site for a potential COVID-19 illness visit. He did not recover from his renal failure.
Subject Dies – January 11, 2021
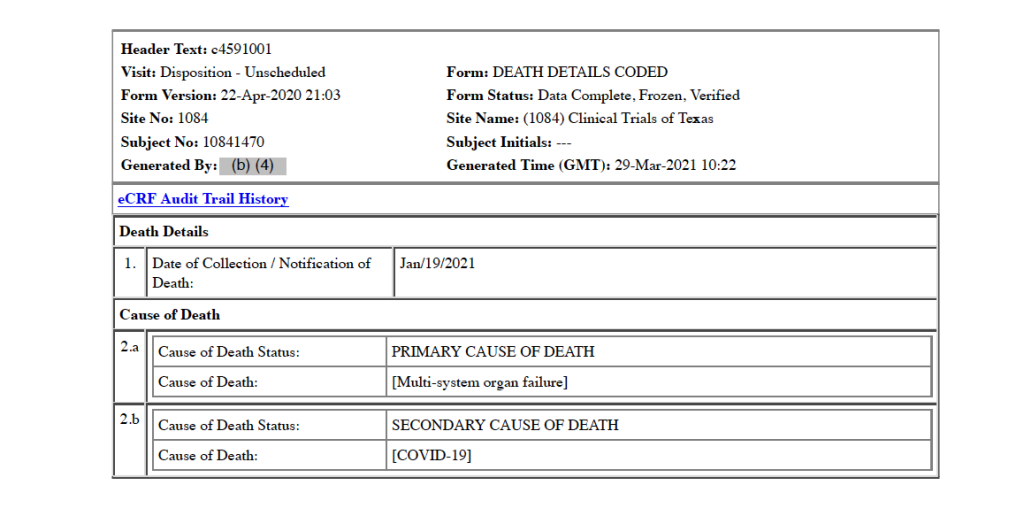
On January 11, 2021, 20 days after receiving Dose 1 of the Moderna mRNA COVID vaccine, Subject 10841470 dies. His primary cause of death is listed as multiple organ dysfunction syndrome, and the secondary cause of death is listed as COVID-19. [pp. 83-84] Prior to his death, vital signs for January 11 are noted as: blood pressure 90/54, respiration of 21 breaths per minute, oxygen saturation level of 96, and heart rate of 132 beats per minute. The CRF states that the subject is discontinued from the study on this date. [p. 82]

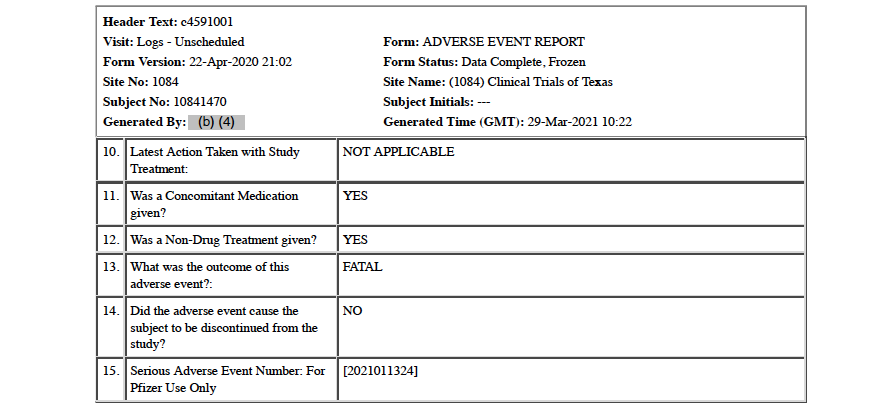
Five medical doctors who are part of the War Room/DailyClout Pfizer Documents Analysis Project reviewed this CRF, and all five disagree with the assigned cause(s) of death. One doctor indicated that COVID-19 should have been listed as the primary cause of death. Another doctor said this is a clear case of Acute Respiratory Distress Syndrome (ARDS) as the cause of death, as well as stating that there is not documentation in the CRF to support diagnoses of acute renal failure or multi-organ dysfunction syndrome.
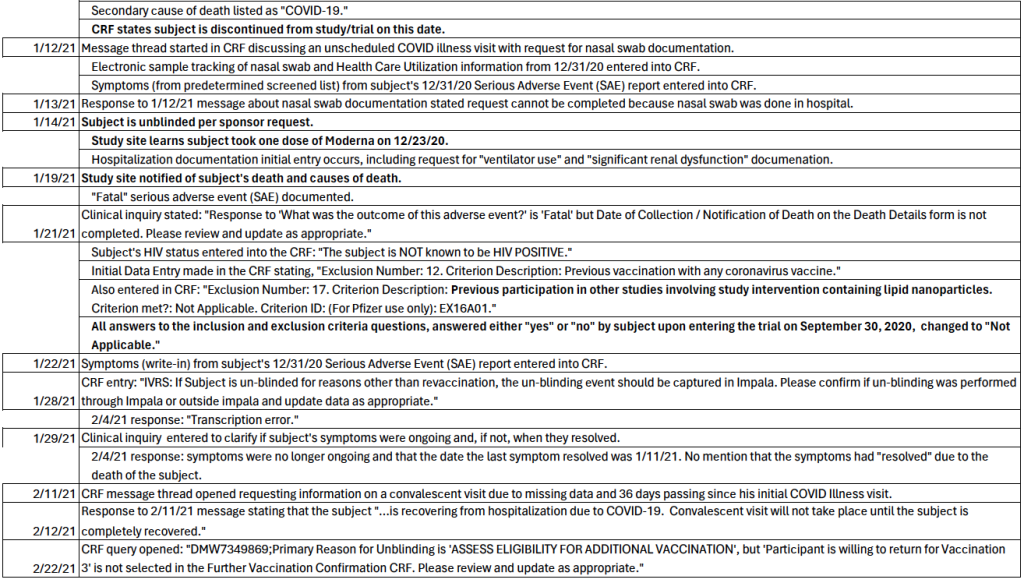
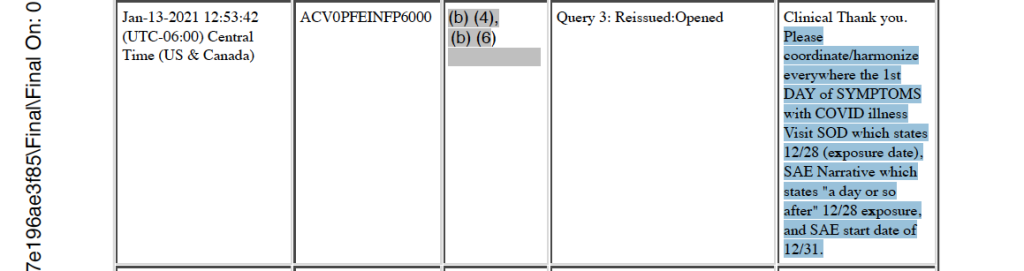
Unscheduled Potential COVID Illness Visit Discussed, SAE Report Symptoms Entered – January 12, 2021, through January 22, 2021
Beginning on January 12, 2021, after the patient’s death, a message thread discussing an unscheduled potential COVID illness visit began with a request for nasal swab documentation. On January 13, 2021, and January 21, 2021, responses were given that this request could not be completed, because the nasal swab was done in the hospital. [p. 221] The discussion thread was closed on January 22, 2021, at 15:16 CST. Electronic sample tracking of the subject’s nasal swab test and Health Care Utilization information from December 31, 2020, are entered into the CRF for the first time on January 12, 2021. [pp. 236-241]
Additionally, symptoms from his Serious Adverse Event (SAE) report completed on December 31, 2020, at the time of his COVID-19 diagnosis with emergency room presentation and subsequent hospital admission, are entered in the subject’s CRF on January 12, 2021, (from a list of pre-determined screened symptoms) and January 22, 2021 (write-in symptoms). [pp. 224-230]
Subject Is Unblinded, Study Site Finds Out He Took Moderna Dose 1 – January 14, 2021
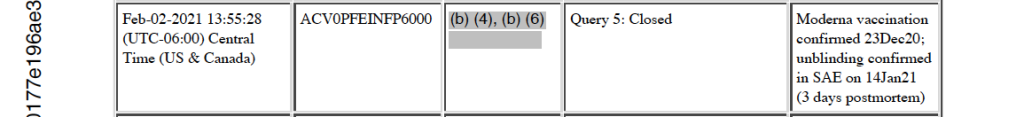
Three days later, on January 14, 2021, deceased Subject 10841470 is unblinded, per sponsor request, and it is noted that this is the date that the study site (1084 in Texas) find out the subject received one dose of the Moderna vaccine on December 23, 2020. [p. 318]
Also on this date, hospitalization documentation initial entry occurred, including the request for the “ventilator use” documentation (response entered on January 21, 2021, at 13:13 CST), along with a request for the “significant renal dysfunction” documentation (response entered on January 22, 2021, at 14:32 CST). [pp. 243-252]
Pfizer Study Site Notified of Subject’s Death, Documents “Fatal” Serious Adverse Event – January 19, 2021
Five days later, on January 19, 20201, the CRF shows that the study site was notified of the subject’s death, as well as the primary and secondary causes of death. [p. 110]
Request for Notification of Death Date, Subject’s HIV Status Documented, Inclusion/Exclusion Answers All Changed to “Not Applicable” – January 21, 2021
At 15:39 CST on January 21, 2021, the CRF shows a clinical inquiry stating: “Response to ‘What was the outcome of this adverse event?’ is ‘Fatal’ but Date of Collection / Notification of Death on the Death Details form is not completed. Please review and update as appropriate.” [pp. 326-327]
Later that day, the subject’s negative HIV status was entered into the CRF: “The subject is NOT known to be HIV POSITIVE.” [p. 197]
Additionally, on this date, an initial Data Entry was made in the CRF stating, “Exclusion Number: 12. Criterion Description: Previous vaccination with any coronavirus vaccine.” [Emphasis Added. p. 175] A staff member also enters, “Exclusion Number: 17. Criterion Description: Previous participation in other studies involving study intervention containing lipid nanoparticles. Criterion met?: Not Applicable. Criterion ID: (For Pfizer use only): EX16A01.” [pp. 185-187] It is unclear what the previous study was that involved lipid nanoparticles.
Moreover, and inexplicably, on this date, all answers to the inclusion and exclusion criteria questions, which the subject answered either “yes” or “no” upon entering the trial on September 30, 2020, were changed to “Not Applicable.”
Was Subject Unblinding for Reasons “Other Than Revaccination” and Captured in Impala? – January 28, 2021
The CRF shows the following entry: “IVRS: If Subject is un-blinded for reasons other than revaccination, the un-blinding event should be captured in Impala. Please confirm if un-blinding was performed through Impala or outside impala and update data as appropriate. Thank you.” [p. 354] An answer came on February 4, 2021, stating, “Transcription Error.” [p. 353]
Are Subject’s Symptoms Ongoing? – January 29, 2021
On January 29, 2021, a clinical inquiry was entered to clarify if the subject’s symptoms were ongoing and, if not, when they resolved. A response came on February 4, 2021, at 14:04 CST, indicating that the symptoms were no longer ongoing and that the date the last symptom resolved was January 11, 2021. However, there was no mention that the symptoms had “resolved” due to the death of the subject. [p. 223]
Convalescent Visit Discussed – February 11, 2021, through March 4, 2021
Starting on the morning of February 11, 2021, one month after the subject’s death and three weeks after his death was entered into the CRF, a message thread opened requesting information on a convalescent visit due to missing data and 36 days passing since the initial COVID Illness visit. A response came on February 12, 2021, at 10:32 CST that the subject “…is recovering from hospitalization due to COVID-19. Convalescent visit will not take place until the subject is completely recovered.” The response was acknowledged on February 15, 2021; and, on March 4, 2021, it was noted that the need for this convalescent visit documentation will be put on hold in the “PD tracker.”
There was never acknowledgement that the subject was already dead.
Confirmation of “Post-Mortem” Unblinding, Three Days After Death, Due to Receipt of Moderna COVID Vaccine – February 22, 2021
In answer to the February 10, 2021, clinical inquiry, “Please submit reason for unblinding (3 days post-mortem) to the SAE in a safety update,” an answer was entered on February 22, 2021, as, “Site was made aware of the fact that subject received Moderna vaccine on 23DEC2020.” [p. 318]
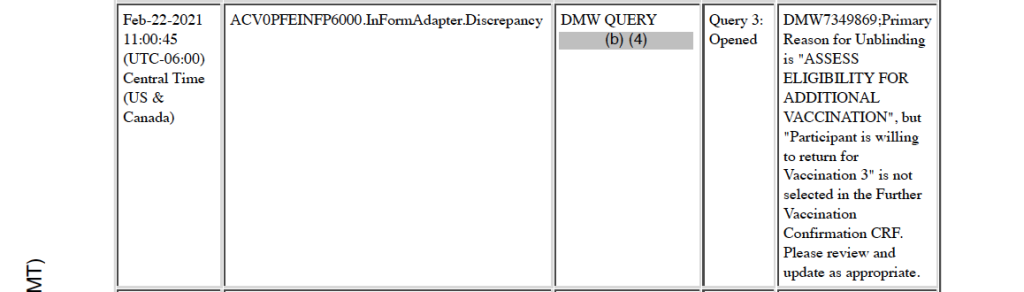
On February 22, 2021, almost one-and-a-half months after the subject’s death, a CRF query opened: “DMW7349869;Primary Reason for Unblinding is ‘ASSESS ELIGIBILITY FOR ADDITIONAL VACCINATION’, but ‘Participant is willing to return for Vaccination 3’ is not selected in the Further Vaccination Confirmation CRF. Please review and update as appropriate.” [p. 353] This entry highlights that serious adverse events (SAEs), such as death, are not readily apparent in the CRF format. A better designed system would not allow a situation such as this to arise in the first place.
Both in life and death, Subject 10841470 experienced a harrowing path through the COVID-19 vaccine clinical trial experience.
Was he informed of trial-prohibited medications, such as the Moderna COVID vaccine? He did not know he was in the placebo cohort, so what made him risk getting a possible third dose of an mRNA COVID vaccine in under three months? Perhaps the never-ending fearmongering by the media, public health officials, and the government, especially around co-morbidities from which he suffered, influenced his decision to seek out the allegedly “lifesaving vaccine?”
Additionally, his receipt of Moderna while in the Pfizer trial highlights the potential unknown contamination of COVID-19 vaccine clinical trials. One cannot know how many subjects received non-Pfizer COVID vaccines and did not disclose them.
Just days after receiving the first dose of the Moderna COVID vaccine, this trial subject contracted COVID which then led to him being hospitalized, ventilated, and having multi-organ failure. Less than three weeks after taking one dose of Moderna’s mRNA-1273, he passed away while still in the hospital. However, his clinical trial journey did not end there.
One day after his death, the study site staff discussed bringing him in for a potential COVID illness site visit. This indicates that they were not aware of his hospitalization and acute illness.
Three days post-mortem, Subject 10841470 was unblinded, and ventilator use, renal dysfunction, and one dose of Moderna vaccine were documented in the CRF. Eight days after his death, the study site found out he died and noted a “fatal” serious adverse event.
What seems to be a CRF clean-up of sorts starts to happen after his passing. Ten days after the subject died, staff entered his HIV status as “not known to be HIV positive.” Inexplicably, on that same day, staff changed all of the subject’s “yes” and “no” inclusion and exclusion criteria answers recorded on September 30, 2020, to “Not Applicable.” After he had been dead 18 days and when the study site was already aware of his death, an inquiry was entered in the CRF asking if his symptoms are ongoing.
The “speed of science” appears to lead to an inability to follow trial protocol correctly, as well as widespread miscommunication among trial personnel and seeming lack of compassion for the suffering and deceased.



