“Pfizer hid vaccine deaths, research team alleges”
During the clinical trials for its COVID-19 vaccine, pharmaceutical giant Pfizer appears to have hidden two deaths — including one in Kansas — which researchers allege would have revealed potentially dangerous side effects to the vaccines.
Over the last year and a half, a team of researchers — volunteering for The Daily Clout, a non-profit news outlet — including physicians, a businessman, and a former United States Army Intelligence officer poured through thousands of pages of documents relating to the study and found that Pfizer had failed to report the deaths of two women — one in Kansas and one in Georgia — during the trial.
Not only did they fail to report them, but they had—in fact—apparently actively covered them up.
According to Dr. Jeyanthi Kunadhasan, an anesthetist and perioperative physician in Australia; who was part of the team, the study protocol required that any “death or serious adverse effect” had to be reported within 24 hours. In the Kansas case that did not happen for 37 days.
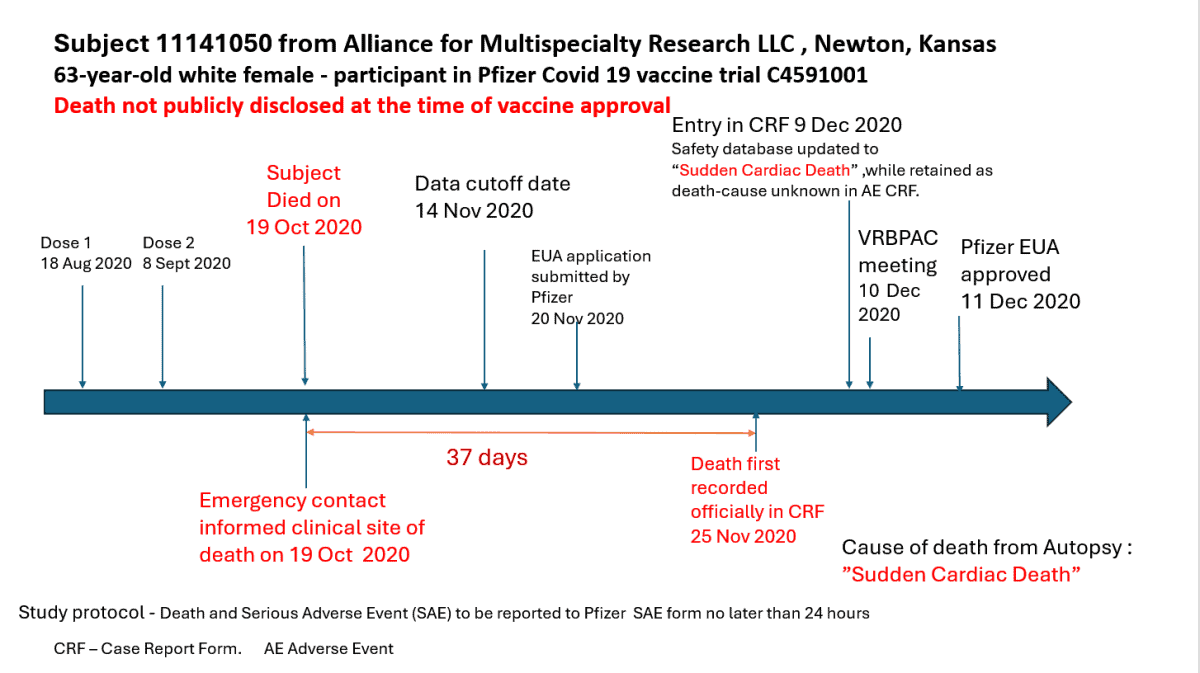
The Kansas case was a 63-year-old woman who had her first dose of the Pfizer mRNA vaccine on August 18, 2020, and a second dose on September 8, 2020. She died on October 19, 2020, and her emergency contact immediately informed the clinical site — Alliance for Multispecialty Research LLC, in Newton, Kansas. Thirty-seven days later, on November 25, 2020 — 11 days after the data reporting cutoff date — the death was finally recorded in a “case report form.”
Five days after the emergency use application was submitted to the Food and Drug Administration by Pfizer.
The participant’s death was not reported in the trial results in the prestigious New England Journal of Medicine or to the FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC), which approved the EUA.
According to a letter Kunadhasan sent to Kansas Attorney General Kris Kobach — who is suing Pfizer over the vaccine — an autopsy recorded the cause as “sudden cardiac death,” and Pfizer physicians ruled her death was not related to the vaccine, citing “risk factors” for heart disease to include hypertension and obesity.
However, according to Kunadhasan, the patient was hardly obese but rather mildly overweight, at approximately 5 feet 4 inches tall and 163 pounds.
“To be eligible for inclusion in this clinical trial, participants had to be deemed healthy based on medical history, physical examination (if required), and the clinical judgment of the investigator. The protocol allowed healthy participants with pre-existing stable disease – defined as disease not requiring significant change in therapy or hospitalization for worsening disease during the six weeks before enrolment – to participate in the clinical trial,” Kunadhasan wrote. “This patient was medicated with two different antihypertensives and had encountered clinical trial personnel at least three times with no mention of any worryingly high blood pressure readings. In fact, I cannot find any blood pressure reading in her publicly available case notes. Consequently, I can only assume the patient’s high blood pressure, from which she had suffered since January 1st, 2010, was well-controlled when she was admitted to the trial.
The patient was 165cm tall and weighed 74.1kg. Hence, her BMI (body mass index) of 27.2 put her in the overweight category, not obese.
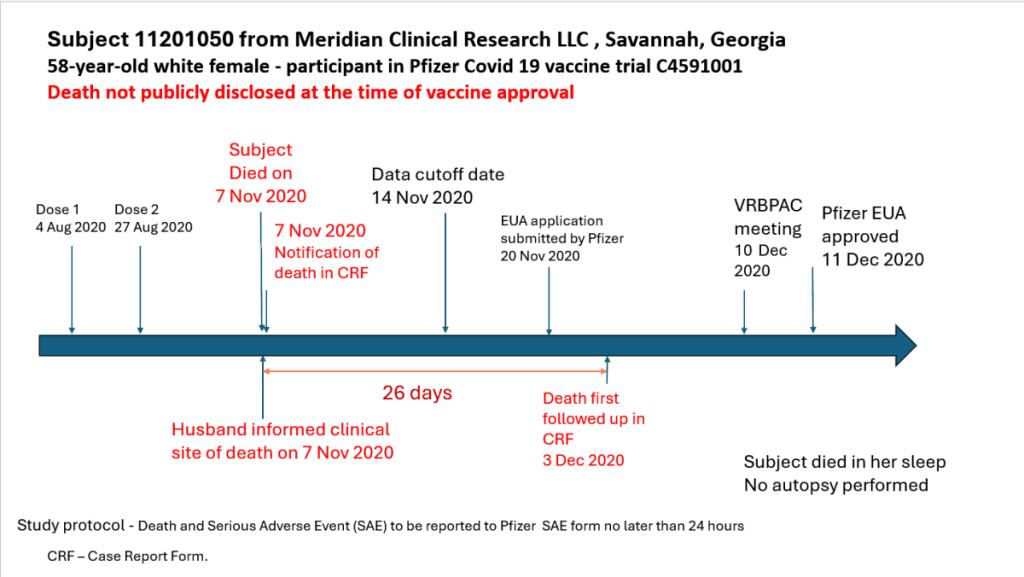
The second hidden death was a 58-year-old woman in Georgia.
Continue Reading
______
Follow DailyClout on Rumble! https://rumble.com/user/DailyClout
Please Support Our Sponsors
Birch Gold Group: “A Gold IRA from Birch Gold Group is the ultimate inflation hedge for your savings in uncertain times. Visit https://birchgold.com/dailyclout to see how to protect your IRA or 401(k).”
The Wellness Company: https://dailyclouthealth.com
Use code DAILYCLOUT for 10% off!
NativePath: “Electrolyte and sports drinks are doing more harm than good to your body. Stay healthy and hydrated with NativeHydrate… Visit https://nativehydrate.com/DailyClout to learn more.”
Patriot Mobile: “Visit https://patriotmobile.com/dailyclout for a FREE month of service when you switch!”
Order ‘The Pfizer Papers’ and Support Our Historic Work: https://www.amazon.com/dp/1648210376?&tag=skyhorsepub-20
Discover LegiSector! Stay up-to-date on issues you care about with LegiSector’s state-of-the-art summarizing capabilities and customizable portals. No researchers needed, no lobbyists, no spin. Legislation at your fingertips! Learn more at https://www.legisector.com/
One of our country’s most important freedoms is that of free speech.
Agree with this essay? Disagree? Join the debate by writing to DailyClout HERE.




