472 Pages FOIAed from CDC Leaders, 2021, Reveal Lengthy Comms Re: ‘Myocarditis,’ Massive Redactions. CDC Hiding April 2021 Monthly Safety Report on Pfizer’s Vaccine and Myocarditis/Pericarditis.

Attorney Edward Berkovich submitted a Freedom of Information Act (FOIA) request to the Centers for Disease Control and Prevention (CDC) stating, “I request emails sent by and received by Dr. Rochelle P. Walensky, Sherri A. Berger, and Kevin Griffis (all of whom are CDC personnel) on dates beginning February 1, 2021 through May 31, 2021, containing the word myocarditis.” [Emphasis added.] [https://www.cdc.gov/about/leadership.htm]
Previously, Mr. Berkovich asked multiple state attorneys general to consider state-level action to investigate Centers for Disease Control and Prevention (CDC) officials for reckless endangerment or similar state crimes for CDC’s three-month delay in reporting the first statistically significant signal of myocarditis incidence following mRNA COVID-19 vaccination. [https://dailyclout.io/letters-to-13-state-attorneys-general-to-consider-investigating-and-prosecuting-cdc-officials-for-reckless-endangerment-or-similar-crimes/]
The 472-page, heavily redacted document production may be read below. (Use the arrows at the bottom of the PDF window to turn the pages.)
- 161 pages are unredacted.
- 153 pages are partially redacted.
- 15 pages are fully redacted.
- Additionally, pages were identified as part of this production and require “consultation…from other government entities.” Those will be released several months from now.
Definitions of the exemptions under the Freedom of Information Act (5 U.S.C. § 552) which apply to this production:
- Exemption 4: Trade secrets or commercial or financial information that is confidential or privileged.
- Exemption 5: Privileged communications within or between agencies, including those protected by the:
- Deliberative Process Privilege (provided the records were created less than 25 years before the date on which they were requested)
- Attorney-Work Product Privilege
- Attorney-Client Privilege
- Exemption 6: Information that, if disclosed, would invade another individual’s personal privacy.
Many of the exemptions do not appear to apply to sections which were redacted with them.
Information from Emails:
- pp. 426-440 – A confidential April 1-29, 2021, Pfizer Summary Monthly Safety Report on Pfizer-BioNTech Vaccine Myocarditis and Pericarditis. Only the cover page is visible. The rest of the report is completely redacted.
p. 21-30 – A May 29, 2021, email from Robert (“Robbie”) Goldstein, MD – Massachusetts’ Commissioner of the Department of Public Health (DPH), a former Senior Policy Advisor at the Centers for Disease Control and Prevention (CDC), an infectious disease physician at Massachusetts General Hospital (MGH), and a faculty member at Harvard Medical School – emailed the May 27, 2021, Journal of American Medical Association (JAMA) Cardiology article, “Prevalence of Clinical and Subclinical Myocarditis in Competitive Athletes With Recent SARS-CoV-2 Infection: Results From the Big Ten COVID-19 Cardiac Registry” article to CDC Director Rochelle Walensky, MD. In the article, 2.3% were positive. Nine were clinically evident, and 28 presented with subclinical myocarditis. - p. 31 – On May 23, 2021, Demetre Daskalakis, MD, COVID CDC Response Role: Senior Lead, Equity in COVID Data and Engagement and Director of CDC Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, emails Henry Walke, MD, MPH, CDC Director of the Office of Readiness and Response, with attached slide deck titled, “Myocarditis update deck 5232021 Final.” The email notes, “Complete w DOD.” That is followed by redacted pages 32 through 44 and then redacted pages 46 through 57.
- Page 45 is an unredacted email from Mr. Daskalakis and indicates that he has “the raw VAERS in here.”
- p. 62 – On May 23, 2021, an email authored by Patrick Caubel, Chief Safety Officer at Pfizer, is forwarded to Rochelle Walensky about “Myocarditis/age group” “latest Pfizer data.” The rest of the email is fully redacted.
- p. 424 – On May 22, 2021, Mr. Caubel from Pfizer, emails a person named Larry saying, “this is the data for MYOCARDITIS (excluding pericarditis). Cut-off date is today. [Redacted.] Number of valid Adverse Event cases reported to Pfizer as of today is [redacted] are myocarditis cases. [Redacted.] Attached our latest monthly aggregate analysis.”
- That is followed by a two-column table – one column is “Month” (January-May 2021) and the other is “#cases received.” The “# of cases” are redacted.
- That is followed by a two-column table – one column is “Country” and the other is “# cases.” “# cases” is redacted.
- A redacted person sends this to Dr. Walensky stating, “The attachment has the Israeli data included. It has all been sent to your team.”
- p. 424 – On May 22, 2021, Mr. Caubel from Pfizer, emails a person named Larry saying, “this is the data for MYOCARDITIS (excluding pericarditis). Cut-off date is today. [Redacted.] Number of valid Adverse Event cases reported to Pfizer as of today is [redacted] are myocarditis cases. [Redacted.] Attached our latest monthly aggregate analysis.”
- p. 78 – A May 16, 2021, email to Christa Capozolla, CDC Global Health Center (GHC) Deputy Director for Strategy, Policy, and Communication, and Sherri Berger, Deputy Director for Policy, Communications, and Legislative Affairs/Chief Strategy Officer, indicates that Deloitte is running the CDC’s Incident Management Systems (IMS) weekly update meetings. Deloitte is asking what topics Ms. Capozolla and Ms. Berger want to cover in the next meeting. Deloitte Services, LP, requested touching on communication items, “three deliverables,” “support, if any, needed at the end of this four-week transition period.”
- p. 93 – On May 20, 2021, Céline Grounder, MD, sent an email with the subject “Q’s re: NYT op ed Jeremy Faust and I are writing (already green lit)” to Rochelle Walensky and John T. Brooks, MD, Chief Medical Officer, CDC COVID-19 Response, stating, the article is “about the risks-benefits of COVID and COVID vaccination, including the risk of long-term side effects of COVID vaccination (low) versus the long-term risks of COVID (high).” She continues noting that Jeremy Faust, MD, are “hearing more reports of post-vaccine myocarditis from MA and NY…as well as elsewhere in the country. Is there anything you can share about post-vaccination myocarditis cases (demographics? fatal / non-fatal?) Or other serious post-vaccination events?” Dr. Gounder concludes, “These data could be just shared with us on background. We just want to make sure that in framing our argument, we’re not misrepresenting the facts.”
- Céline Grounder was married to Grant Wahl, the popular CBS Sports Soccer Analyst, senior Sports Illustrated writer, and Fox Sports correspondent, who died suddenly in December 2022 of a ruptured aortic aneurysm while covering the Qatar World Cup.
- p. 237 – On May 20, 2021, Dr. Céline Gounder also tells Dr. Walensky and Dr. Brooks, “We’re hearing more and more reports of post-vaccination myocarditis in MA and NY where Jeremy and I are based, as well as elsewhere across the country.” [Emphasis added.]
- pp. 95-97 – Dr. Gounder’s email includes a three-page letter from Jeremy Faust, MD, to a Dr. Brooks. “Twice now, the New York Times’ David Leonhardt has propagated a CDC statistic that is dubious and potentially harmful.” The statistic of concern is that COVID-19 is “no more harmful to children than seasonal influenza.” Dr. Faust goes on to dispute this and takes issue with “two staffers at NCIRD” [National Center for Immunization and Respiratory Diseases ]. He concludes, “The CDC should resolve this issue by instructing the NCIRD to remove estimated influenza deaths from its reporting.” [Emphasis added.] There is no evidence of a response to the letter.
- p. 179 – On May 21, 2021, Dr. Brooks emails former CDC Director, Rochelle Walensky, MD, and says, “I should have asked whether in addition to reaching out about myocarditis (had a good call with Celine [Gounder, MD] and updated her on what can be shared publicly), did you also [large section redacted].” [Emphasis added.]
- Pg 236 – On May 21, 2021, Anne Schuchat, MD, Former Deputy Director of the CDC and GAVI Board Member, emails team about the “green lit” New York Times article being co-authored by Céline Gounder, MD, and Jeremy Faust, MD. “New Guidance on Booster Shots Gets Ahead of the Science” was published on September 24, 2021, but not with Gounder’s byline. Instead, it had a byline for Dr. Faust and Megan L. Ranney, MD, who was not part of the email thread. There appears to be collaboration between the CDC and NYT to push a certain narrative.
- pp. 101-106 – May 14, 2021, There are two emails regarding “vaccines and myocarditis” with the second, from Thomas A. Clark, MD, Deputy Division Director of Division of Viral Diseases, to Dr. John Brooks, stating “Thanks, John. I’ll just reinforce that initiating VAERS reports is the best way to get information to us—these cases are escalated and VAERS will reach out for more information and to collect medical records. Also asking Tom the best approach to organizing a call—it might be to initiate a CISA [Cyber Infrastructure Security Agency] consult and include involved providers, but he may wish to do something sooner and less formal for now since it sounds like several institutions are involved.”
- p. 102 – Those emails are followed by a May 14, 2021, email from Dr. Brooks stating, “Looping everyone in with Matt Oster, pediatric cardiologist at Emory and also with CDC (chronic disease). He shared the following info below. Very willing to work with whomever he might best connect in the vaccine safety group.”
- That is followed by another May 14, 2021, email from Dr. Oster that starts “Updates:” and then has the first five update items of the email are redacted.
- p. 102 – Those emails are followed by a May 14, 2021, email from Dr. Brooks stating, “Looping everyone in with Matt Oster, pediatric cardiologist at Emory and also with CDC (chronic disease). He shared the following info below. Very willing to work with whomever he might best connect in the vaccine safety group.”
- p. 109 – On May 14, 2021, Dr. Brooks tells Matt Oster, MD, Director, Children’s Cardiac Outcomes Research Program at Sibley Heart Center (CORPS) at Children’s Healthcare of Atlanta, Cardiology, that “Although Israel, European Medicines Agency and US DoD are investigating the issue at present, a clear association has not yet been established. As such, the topic was not included in discussions during the recent ACIP meeting. However, this Monday (May 17) the ACIP subgroup that routinely reviews data for safety signals is going to be discussing this topic.”
- p. 111 – On May 11, 2021, Dr. Brooks is in a discussion with Dr. Oster about myocarditis in young men and whether or not Dr. Oster should speak at the upcoming Advisory Meeting on Immunization Practices (ACIP) meeting. Dr. Brooks responds to Dr. Oster saying, “Righto – I know the topic has been much discussed by the vaccine safety folks and I believe I believe the ACIP safety group. Curious if it [comes] up at tomorrow’s meeting. They certainly are not aware. 😉”
- p. 196 – On May 14, 2021, Jane W. Newburger, MD, Associate Cardiologist-in-Chief for Academic Affairs at Children’s Hospital Boston, emails Amanda Cohn, MD, CAPT, USPHS Director, Division of Birth Defects and Infant Disorders; Mary Beth Son, MD, Section Chief, Rheumatology Program; Director, Samara Jan Turkel Clinical Center; Director, Services and Outreach and Associate Professor of Pediatrics, Harvard Medical School; and Sarah Mbaeyi, MD, MPH, CDC staff member and doctor at Children’s Healthcare of Atlanta saying, “I’m writing to keep you in the loop. As we anticipated, the post-vaccine myocarditis events have risen to the attention of leaders at our institution (this is also a topic of conversation among Chief Safety Officers at Children’s hospitals). Mary Beth and I were emailed today by our hospital Emergency Management, stating, ‘Our hospital is putting together messaging for clinicians to provide information on myocarditis in COVID-vaccinated individuals and give direction on what to do if they think their patient is a case‘ and asking for direction about to whom children should be referred. We sent out the information re: direction of case to the CDC sites. Do you think the CDC will be having any sort of official statement about this soon? Or will it have to wait till analysis of cases? This is just for information for our hospital officials – I realize it is very unlikely for you to have a statement so soon.” [Emphasis added.]
- pp. 199-206 – On March 12, 2021, Michael J. Beach, PhD – Principal Deputy Incident Manager, CDC COVID-19 Emergency Response; CDC Spokesperson; and Mahoning County (Ohio) Public Health Associate Director for Healthy Water, National at the Center for Emerging, Zoonotic, and Infectious Diseases – emailed Sherri Berger about “COVID-19: CDC/HHS Product Awareness” saying “Discussion document for today.” The attachment is titled, “Weekly CDC Forecasting of Materials for HHS Awareness Week of 03-15-2021” and is fully redacted other than that title.
- pp. 207-208 – Mid-May 2021 emails involving Sherrie Bruce, Public Health Program Specialist at the CDC who appears to have been very involved as a Chief of Staff in the 2014-2016 Ebola epidemic in West Africa. The emails, including the subject lines, are redacted to the point of being uninterpretable.
- pp. 242-243 – May 24, 2021, emails between Marissa Padilla, Senior Vice President, Communications & Public Affairs at Global Strategy Group and former Biden-Harris Transition Team Volunteer; Abbigail Tumpey, former Associate Director for Communication Science for CDC’s Public Health Infrastructure and now Vice President, Institute Communications at Georgia Institute of Technology; Sherri Berger; and Dr. Goldstein, about “Governors Call TPs” with an attachment named, “Reactive on myocarditis and Tough QA_05232021 Mp.docx.”
- The May 25, 2021, Morbidity and Mortality Weekly Report is mentioned saying, “Topline on MMWR tomorrow (website:” and then the rest is redacted.
- The emails also mention “Reactive on myocarditis” and then has the related information redacted.
- Dr. Goldstein writes to Ms. Tumpey indicating that he is “pulling together talking points for Rochelle’s 10-15 minutes with the Governors tomorrow. Was thinking of the following, but wanted to make sure it fits with your goals for Comms this week…[redacted section]…I haven’t seen any specific asks from the WH or the Governors.”
- p. 263 – an email dated May 24, 2021, from Dr. Goldstein to Dr. Walensky also relates to “Governors Call TPs.” Dr. Goldstein states, “Attached are talking points for tomorrow’s call with the Governors – starting with key metrics, talking briefly about breakthroughs (related to MMWR for tomorrow), and then closing with myocarditis. I included Q&As at the end in case you want them for reference.” The rest of the email is fully redacted.
- pp. 250-252 – May 21, 2021, emails about “WA DOH to Issue a HAN [Health Action Network] alert.”
- The email thread starts with Jennifer Byrne, MPH, Task Force Liaison (TF LNO) Team Lead, Health Department Section, State, Tribal, Local & Territorial Support Task Force, COVID-19 Emergency Response, emailing Nicole Fehrenbach, Deputy Division Director, Division of Congenital and Developmental Disorders; and Ramona (“Mona”) Byrkit, Lead, Health Department Section, State, Tribal, Local and Territorial Support Task Force, COVID 19, Anita Pullani, Team Lead COVID-19 Response Task Force, and Leslie Harrison, MD, CDC Epidemiologist to alert them that “WA is likely to issue a HAN alert on this: [redacted].”
- Margaret (“Peggy”) Honein, PhD, MPH, CDC Director, Division of Preparedness and Emerging Infections, loops in several other previously mentioned people, as well as Athalia Christie, Acting Principal Deputy Director, CDC Global Health Center (GHC) and Dale Rose, PhD, MSc, Deputy Director, CDC Division of Bacterial Diseases.
- The CDC describes a Health Alert Network (HAN) as the “CDC’s primary method of sharing cleared information about urgent public health incidents with public information officers; federal, state, territorial, tribal, and local public health practitioners; clinicians; and public health laboratories. CDC’s HAN collaborates with federal, state, territorial, tribal, and city/county partners to develop protocols and stakeholder relationships that will ensure a robust interoperable platform for the rapid distribution of public health information. CDC’s HAN is a strong national program that provides vital health information and the infrastructure to support dissemination at state and local levels. The majority of the state-based HAN programs have more than 90% of their populations covered under the HAN umbrella. The HAN messaging system directly and indirectly transmits Health Alerts, Advisories, Updates, and Info Services to more than one million recipients.” [Emphasis added.]
- The email thread starts with Jennifer Byrne, MPH, Task Force Liaison (TF LNO) Team Lead, Health Department Section, State, Tribal, Local & Territorial Support Task Force, COVID-19 Emergency Response, emailing Nicole Fehrenbach, Deputy Division Director, Division of Congenital and Developmental Disorders; and Ramona (“Mona”) Byrkit, Lead, Health Department Section, State, Tribal, Local and Territorial Support Task Force, COVID 19, Anita Pullani, Team Lead COVID-19 Response Task Force, and Leslie Harrison, MD, CDC Epidemiologist to alert them that “WA is likely to issue a HAN alert on this: [redacted].”
- p. 257 – On May 26, 2021, Abbigail Tumpey sends an email to Dr. Walensky with Dr. Goldstein, Sherri Berger, and Jason McDonald, a CDC spokesperson, CCed. The subject is “DRAFT HAN – still in clearance” with the attachment, “DRAFT Brief Myocarditis HAN 5.25.2021 to JIC – clean.docx.” The rest of the email is redacted.
- pp. 267-270 – May 13-14, 2021, email thread about “Adolescent vaccine update.”
- On May 13, 2021, Amanda Cohn emails Sam Posner, PhD, Acting Director, National Center for Immunization and Respiratory Diseases (NCIRD); Dana Meaney-Delman, MD, CDC Lead, Maternal Immunization; CDC Chief of Infant Outcomes Monitoring Research and Prevention Branch, Fellow of the American College of Gynecology; and Henry Walke, with many others CCed, stating, “One follow-up. Peter Marks [Director – Center for Biologics Evaluation and Research (CBER) at FDA] called me about something else and we discussed [Adolescent vaccine update] briefly. He agreed communications around this is [sic] incredibly challenging and this is not the same as TTS. Once we decide if we are putting anything out to providers, he would like to see a draft in advance.” [Emphasis added.]
- Henry Walke sends another May 13, 2021, email in this thread saying “FYI, following up with VTF [Vaccine Task Force] to for [sic] option on way forward,” and almost the entire email, from Amanda Cohn, that he forwarded is redacted.
- p. 271-272 – On May 14, 2021, Kristen Nordlund, Health Communication Specialist at CDC, forwards an email from NCIRD Immunization Grantee Mailbox (CDC) with the subject “For Awareness: Monitoring Reports of Myocarditis” to Abbigail Tumpey; Lorrie McNeill, Director, FDA Office of Communication, Outreach and Development; and Ian Sams, former Health and Human Services [HHS] Deputy Assistant Secretary for Public Affairs, COVID Response and current Special Assistant to the President and Senior Advisor and Spokesman for the White House. The email says:
In recent weeks, there have been reports of myocarditis occurring after COVID-19 vaccination, including in Europe, where the EMA recently requested data from Pfizer and Moderna on reports of myocarditis and pericarditis after vaccination. CDC is aware of these reports, which are rare given the number of vaccine doses administered, and continues to monitor available data.
Myocarditis is the inflammation of the heart muscle and pericarditis is the inflammation of the lining outside the heart. In both cases, the body’s immune system is causing inflammation in response to an infection or some other trigger. While myocarditis can be serious, it is frequently mild and self-limited. Symptoms can include abnormal heart rhythms, shortness of breath, or chest pain.
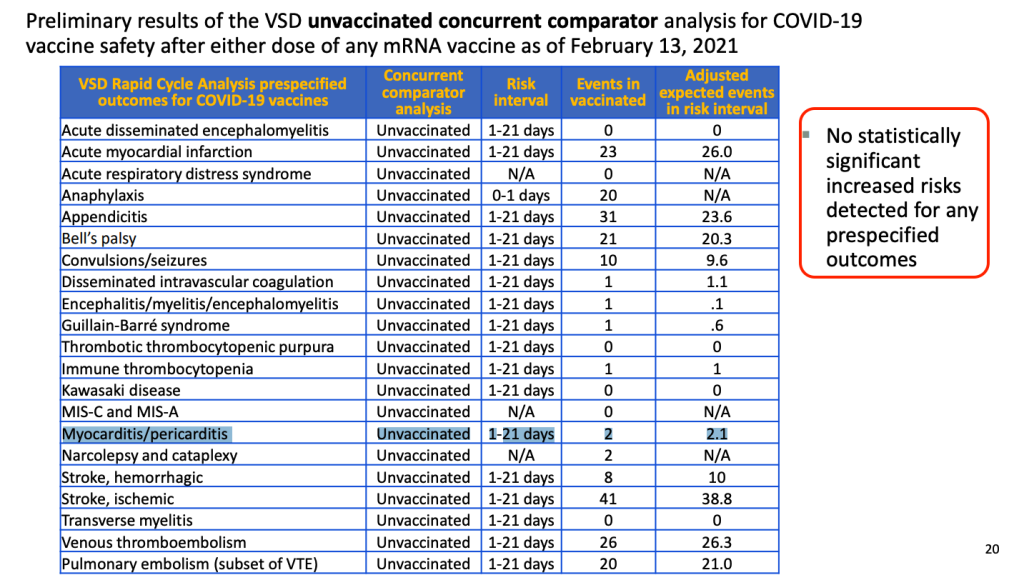
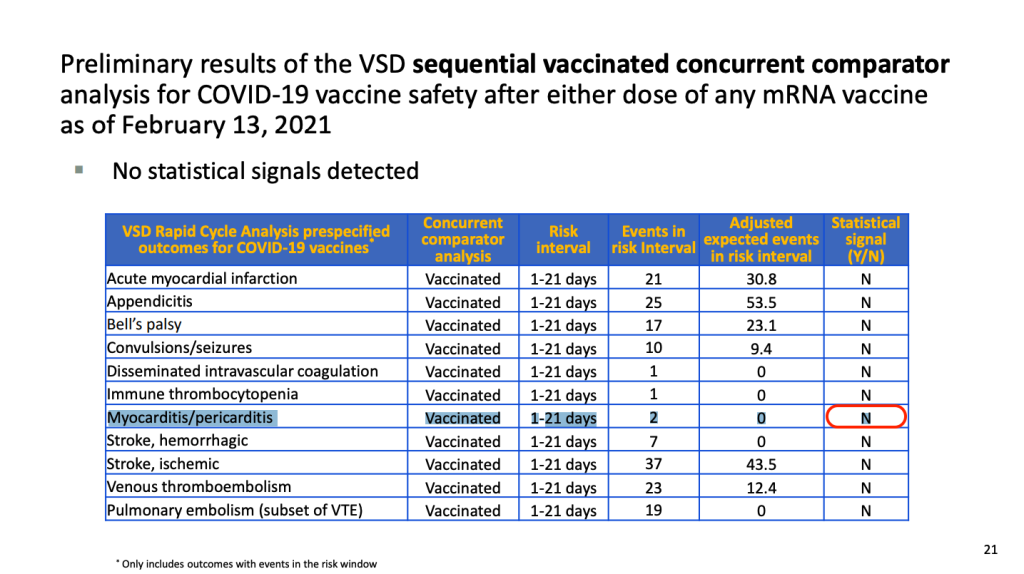
As part of COVID-19 vaccine safety efforts, we have been closely monitoring myocarditis/pericarditis in multiple safety systems, including the Vaccine Adverse Event Reporting System (VAERS) and the Vaccine Safety Datalink (VSD).
To date, there has not been a safety signal identified in either VAERS or VSD. CDC will continue to evaluate reports of myocarditis/pericarditis occurring after COVID-19 vaccination and will share more information as it becomes available. Healthcare providers should consider myocarditis in an evaluation of chest pain after vaccination and report all cases to VAERS.
CDC continues to recommend COVID-19 vaccination for people 12 years and older.
- pp. 273-275 – May 14, 2021, email thread between Capt. David L. Fitter, MD, CDC Director, Division of Global Migration and Quarantine; Tom Shimabukuro, MD, MPH, MBA, Vaccine Safety Team, CDC COVID-19 Vaccine Task Force; Rebecca Greco Krone, CDC Co-Lead, Vaccine Task Force, COVID-19 Response; and John Su, MD, PhD, MPH, Vaccine Safety Team, CDC COVID-19 Vaccine Task Force, about “Reports re myocarditis.”
- Dr. Shimabukuro sends an email to Dr. Fitter and Dr. Su stating, “We have been monitoring myocarditis/pericarditis as a VAERS adverse event of special interest from the beginning. All these case reports are reviewed and attempts are made to follow-up to collect medical records. These cases are broken down by total identified via an automated search strategy, confirmed reports, and reports under review. We are in the process of updating the status on these reports and potentially reaching out to gather additional information on selected pediatric cases if necessary to prepare for an ACIP VaST [Vaccine Safety Technical] call on Monday.” [Emphasis added.]
- There was a Monday, May 17, 20221, COVID-19 VaST Work Group Report issued. In this meeting, the following was discussed:
- Dr. Shimabukuro sends an email to Dr. Fitter and Dr. Su stating, “We have been monitoring myocarditis/pericarditis as a VAERS adverse event of special interest from the beginning. All these case reports are reviewed and attempts are made to follow-up to collect medical records. These cases are broken down by total identified via an automated search strategy, confirmed reports, and reports under review. We are in the process of updating the status on these reports and potentially reaching out to gather additional information on selected pediatric cases if necessary to prepare for an ACIP VaST [Vaccine Safety Technical] call on Monday.” [Emphasis added.]
“The VaST session on May 17, 2021, included several presentations on myocarditis following mRNA vaccines, from the Department of Defense (DoD), the Vaccine Adverse Event Reporting System (VAERS), and Vaccine Safety Datalink (VSD). There were also brief updates from the Veterans’ Administration (VA) and the Clinical Immunization Safety Assessment (CISA) groups about their plans for future investigation of myocarditis.
VaST concluded that there are relatively few reports of myocarditis to date and that these cases seem to occur:
– predominantly in adolescents and young adults,
– more often in males than females,
– more often following dose 2 than dose 1, and
– typically within 4 days after vaccination. Most cases appear to be mild, and follow-up of cases is ongoing.
Within CDC safety monitoring systems, rates of myocarditis reports in the window following COVID-19 vaccination have not differed from expected baseline rates. However, VaST members felt that information about reports of myocarditis should be communicated to providers.
VaST discussed:
– Further information should be collected through medical record review about potential myocarditis cases that were reported into VAERS.
– Information about this potential adverse event should be provided to clinicians to enhance early recognition and appropriate management of persons who develop myocarditis symptoms following vaccination.
– Collaboration between infectious diseases, cardiology, and rheumatology specialists is needed to provide guidance on diagnosis, treatment, and management of myocarditis.”
-
- Dr. Su then sends a chart of “Reports of myopericarditis identified in VAERS” by “Age group.” The chart is redacted except for the column headers.
- p. 287 – May 27, 2021, email from Ms. Nordlund to Stephanie Caccomo, then FDA Media Relations Director and Ms. McNeill marked with “High” importance and the subject, “Updated myocarditis documents,” states, “Stephanie and Lorrie, Thank you for jumping on the phone with us earlier this afternoon to talk through the language around aftercare…” [emphasis added] with the rest redacted.
- pp. 288-289 – On May 23, 2021, Ms. Tumpey emails Dr. Walensky with the subject “Short media appearance.” The email says, “Rochelle, Here is a short media statement that people are reviewing,” and the rest is redacted.
- Dr. Walensky responds that same day, but the only unredacted phrases are “A quick thought -” and “I think.”
- Ms. Tumpey responds with, Got it.”
- Dr. Walensky responds that same day, but the only unredacted phrases are “A quick thought -” and “I think.”
- pp. 290-291 – On May 21, 2021, Dr. Daskalakis emailed Dr. Walke and Dr. Walensky, and the subject of the email was, “Quick Follow ups from 830: Myocarditis and Retail Pharmacy Updates.” The email is fully redacted other than his signature block.
- Dr. Walke responds that same day, and his response is redacted. He emails once more and adds Dr. Schuchat to the email.
- pp. 294-297 – April 26, 2021, through April 27, 2021, Nicole Coffin, CDC Senior Advisor, Strategic Policy, Communications, and Partnerships emailed Denise Cardo, MD, CDC Director of the Division of Healthcare Quality Promotion (DHQP), National Center for Emerging and Zoonotic Infectious Diseases (NCEZID); Seth Kroop, CDC Associate Director for Policy, Division of Healthcare Quality Promotion; and Kerri Moran, then Healthcare Communications Specialist (with others CCed). The was marked with “High” importance, and the subject of the email was “FW: Myocarditis,” and it said, “FYSA [For Your Situational Awareness], in case asked in other channels. Below is cleared response from Tom and VEU that Abbigail Tumpey will use to respond to a question” and the rest is redacted except for two URLs. Those are:
- https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-02/28-03-01/05-covid-Shimabukuro.pdf – A March 1, 2021, slide deck by Dr. Tom Shimabukuro titled, “COVID-19 vaccine safety update, Advisory Committee on Immunization Practices (ACIP).” It mentions myocarditis/pericarditis twice:
-
- https://www.cdc.gov/mmwr/volumes/70/wr/mm7008e3.htm – A Morbidity and Mortality Weekly Report for “First Month of COVID-19 Vaccine Safety Monitoring — United States, December 14, 2020–January 13, 2021.” It does not mention myocarditis.
- Dr. Cardo responded to Tom Clark and Tom Shimabukuro, changing the subject line to say, “”Please read: myocarditis” and saying, “Tom C and Tom S, Please provide few bullets to CDC (as FYI) about the DoD info on myocarditis/pericarditis. I think it is…” [emphasis added], and the rest is redacted.
- Dr. Clark responded stating, “Thanks Denise. Asking Tom to add a few bullets about DoD findings.” [Emphasis added.]
- p. 300 – On May 28, 2021, Ms. Berger emailed Grace Kwak, White House Advisor to the Deputy COVID-19 Response Coordinator, about “Myocarditis Materials,” and sent two URLs:
- “Public Content” – https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html (“Myocarditis and Pericarditis After mRNA COVID-19 Vaccination”)
- “Clinical Considerations” – https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html (“Clinical Considerations: Myocarditis and Pericarditis after Receipt of COVID-19 Vaccines Among Adolescents and Young Adults”)
- pp. 301-302 – On April 19, 2021, Dr. Meany-Delman emailed Dr. Shimabukuro and Dr. Clark, with others CCed, about “Pericarditis and Mrna vaccines,” stating, “Anne S. is on the deputies call with WH right now and is asking if there has been any signal with pericarditis and mRNA vaccines.”
- Dr. Shimabukuro responded saying, “DoD and Israeli MOH think they have a signal for myocarditis with mRNA vaccines, but there is potentially a lot of ascertainment bias in the DoD data. We don’t have any evidence to suggest a signal or a safety problem for myocarditis or pericarditis with mRNA vaccines from VAERS and VSD surveillance and FDA and VA have not detected any signals in the monitoring. DoD has submitted a case series of myocarditis following mRNA vaccines, so that might be published sometime in the future.”
- Dr. Walensky responded with, “Super helpful. Thank you!”
- Dr. Shimabukuro responded saying, “DoD and Israeli MOH think they have a signal for myocarditis with mRNA vaccines, but there is potentially a lot of ascertainment bias in the DoD data. We don’t have any evidence to suggest a signal or a safety problem for myocarditis or pericarditis with mRNA vaccines from VAERS and VSD surveillance and FDA and VA have not detected any signals in the monitoring. DoD has submitted a case series of myocarditis following mRNA vaccines, so that might be published sometime in the future.”
- p. 304 – On May 14, 2021, Ms. Tumpey forwarded an email from Ms. Nordlund to Dr. Walensky and Dr. Schuchat about “FYI: Myocarditis email.” She stated, “Per our discussion. Coordinating strategy.”
- Ms. Nordlund’s forwarded email says, “CDC plans” and the rest is redacted.
- pp. 305-307 – On April 28, 2021, Dr. Walensky emailed Ms. Tumpey about “Myocarditis TPs [Talking Points],” writing, “We need this for the presser – we [sic] are we in [sic] running this down?” The rest is redacted.
- Ms. Tumpey responds with, “Rochelle, Below is a response and at the bottom are internal notes from the discussions with DoD. Please let me know if you need more. BLUF: [redacted]. Response: [redacted]. Notes from the DoD discussion (not for public release, but for your awareness) [redacted]. Questions and discussion [redacted].” [Emphasis added.]
- Dr. Walensky responded to Ms. Tumpey with, “Very helpful…so presumably [redacted] Do you have any update on what they are finding/doing in Israel?”
- Ms. Tumpey replies, “The vaccine safety team is working [redacted]. They are working with the DoD on this now. Re: Israel – they are doing continued surveillance. [Redacted] Please let me know if you need more.”
- Dr. Walensky replied, “Great – thanks, so [redacted.] Thank you for running this down.”
- Ms. Tumpey replies, “The vaccine safety team is working [redacted]. They are working with the DoD on this now. Re: Israel – they are doing continued surveillance. [Redacted] Please let me know if you need more.”
- Dr. Walensky responded to Ms. Tumpey with, “Very helpful…so presumably [redacted] Do you have any update on what they are finding/doing in Israel?”
- Ms. Tumpey responds with, “Rochelle, Below is a response and at the bottom are internal notes from the discussions with DoD. Please let me know if you need more. BLUF: [redacted]. Response: [redacted]. Notes from the DoD discussion (not for public release, but for your awareness) [redacted]. Questions and discussion [redacted].” [Emphasis added.]
- pp. 307-308 – On April 19, 2021, Dr. Shimabukuro sent an email to Dr. Meaney-Delman, Dr. Cohn, and Sara Elizabeth Oliver, MD, MSPH, Medical Officer, National Center for Immunizations and Respiratory Diseases (NCIRD) and Lead for the COVID-19 Vaccines ACIP Work Group about “RE: Pericarditis and Mrna vaccines” stating, “We reviewed the VSD RCA data this morning and discussed the DoD data. We have substantial doses administered in younger age groups in VSD and don’t have a hint of a signal; it’s actually protective in the vaccinated concurrent comparator. We aren’t really sure why DoD thinks they have a signal?”
- The email gets forwarded to Dr. Schuchat and Dr. Walke, and then Dr. Schuchat sends it to Dr. Walensky saying, “More reassuring.”
- Dr. Walensky responded stating, “Much – great – thank you! Henry – [redacted] we should [redacted]. Thank you!”
- Dr. Walke replies, “Yep on it.”
- Dr. Walensky responded stating, “Much – great – thank you! Henry – [redacted] we should [redacted]. Thank you!”
- The email gets forwarded to Dr. Schuchat and Dr. Walke, and then Dr. Schuchat sends it to Dr. Walensky saying, “More reassuring.”
- p. 311 – On May 24, 2021, Dana Ohannessian of the Massachusetts Department of Health sent an email with the subject “COVID-19 Vaccine Safety Message from CDC via Massachusetts Department of Public Health” to Dr. Walensky saying:
“URLs included to cut and paste into browsers should you choose to avoid clicking on embedded links. MA DPH has received the following communication from CDC:
In recent weeks, there have been reports of myocarditis occurring after COVID-19 vaccination, including in Europe, where the EMA recently requested data from Pfizer and Moderna on reports of myocarditis and pericarditis after vaccination – https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee–prac-3-6-may-2021
CDC is aware of these reports, which are rare given the number of vaccine doses administered and continues to monitor available data.
Myocarditis is the inflammation of the heart muscle and pericarditis is the inflammation of the lining outside the heart. In both cases, the body’s immune system is causing inflammation in response to an infection or some other trigger. While myocarditis can be serious, it is frequently mild and self-limited. Symptoms can include abnormal heart rhythms, shortness of breath, or chest pain.
As part of COVID-19 vaccine safety efforts, we have been closely monitoring myocarditis/pericarditis in multiple safety systems, including the Vaccine Adverse Event Reporting System (VAERS) – https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vaers/index.html and the Vaccine Safety Datalink (VSD) – https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vsd/index.html.
To date, there has not been a safety signal identified in either VAERS or VSD. CDC will continue to evaluate reports of myocarditis/pericarditis occurring after COVID-19 vaccination and will share more information as it becomes available. Healthcare providers should consider myocarditis in an evaluation of chest pain after vaccination and report all cases to VAERS – https://vaers.hhs.gov/reportevent.html.
CDC continues to recommend COVID-19 vaccination for people 12 years and older.”
- p. 312 – On May 24, 2021, Dr. Daskalakis emailed Dr. Walke about “Data by midweek question from RW.” He wrote, “From safety: We will have additional data by mid-week, but I’m not [redacted].”
- p. 313 – On May 24, 2021, Dr. Goldstein sent Dr. Walensky an email about “Governors Call TPs” stating, “Attached are talking points for tomorrow’s call with the Governors – starting with key metrics, talking briefly about breakthroughs (related to the MMWR for tomorrow), and then closing with myocarditis. I included the Q&As at the end in case you want them for reference. One note -tomorrow we will document that 50% of adults are fully vaccinated in the country. Those numbers won’t be released until after the call, but it will come out during the Press Briefing and thought you could foreshadow during this call, as well.”
- No talking points were attached.
- p. 314 – On April 28, 2021, Dr. Walensky emailed Dr. Meaney-Delman, “Subject: Myocarditis,” indicating, “On the deputies call there was [redacted].”
- Dr. Meaney-Delman responded, “Let me ask the safety team. You saw the summary from yesterday, right?”
- Dr. Walensky’s response is mostly redacted.
- Dr. Meaney-Delman responded, “Let me ask the safety team. You saw the summary from yesterday, right?”
- p. 315 – Email thread about “DRAFT_Myocarditis_Advisory_0523201_1109” started by Dr. Walke to Dr. Walensky saying, “Latest HAN.”
- Dr. Walensky responded, “Thank you – Will it be a full HAN – I’m fine with how this reads…grateful.”
- Dr. Walke responded, “Perfect, will push it through. I think Health advisory, [redacted].”
- Dr. Walensky responded, “Thank you – Will it be a full HAN – I’m fine with how this reads…grateful.”
- p. 316 – On May 29, 2021, Dr. Goldstein sends Dr. Walensky the JAMA Cardiology article, “Prevalence of Clinical and Subclinical Myocarditis in Competitive Athletes With Recent SARS-CoV-2 Infection: Results From the Big Ten COVID-19 Cardiac Registry.” [Emphasis added.]
- p. 319 – On May 23, 2021, Dr. Walke emails Dr. Walensky an April 23rd slide deck by Dr. Oliver titled, “Risk/Benefit assessment of thrombotic thrombocytopenic events after Janssen COVID-19 vaccines: Applying Evidence to Recommendation Framework” to ensure Walke and Walensky “are synced.”
- pp. 396-423 – On May 15 and 16, 2021, an email thread between Dr. Walke and Dr. Walensky, with others CCed, titled, “DIRECTORS BRIEF 20210516” has an attached named, “(FOUO) CDC COVID-19 RESPONSE UPDATE – DIRECTORS BRIEF 20210516.pdf.” It is mostly redacted. On page 402, a section reads,
-
- “Monitoring Reports of Myocarditis:
- In recent weeks, there have been reports of myocarditis occurring after COVID-19 vaccination, including in Europe, where the EMA recently requested data from Pfizer and Moderna on reports of myocarditis and pericarditis after vaccination. CDC is aware of these reports, which are rare given the number of vaccine doses administered, and continues to monitor available data.
- Myocarditis is the inflammation of the heart muscle and pericarditis is the inflammation of the lining outside the In both cases, the body’s immune system is causing inflammation in response to an infection or some other trigger. While myocarditis can be serious, it is frequently mild and self-limited. Symptoms can include abnormal heart rhythms, shortness of breath, or chest pain.
- As part of COVID-19 vaccine safety efforts, we have been closely monitoring myocarditis/pericarditis in multiple safety systems, including the Vaccine Adverse Event Reporting System (VAERS) and the Vaccine Safety Datalink (VSD).
- To date, there has not been a safety signal identified in either VAERS or CDC will continue to evaluate reports of myocarditis/pericarditis occurring after COVID-19 vaccination and will share more information as it becomes available. Healthcare providers should consider myocarditis in an evaluation of chest pain after vaccination and report all cases to VAERS.
- CDC continues to recommend COVID-19 vaccination for people 12 years and older.” [This is repeated on pp. 417-418.]
- “Monitoring Reports of Myocarditis:
-
- pp. 449-450 – An email that appears to be from May 27, 2021, contains a section titled, “STLT [State, Local, or Territorial] Support TF.” Its sub-bullets state:
- “STLT’s Health Department and Deployment Sections are working quickly to mobilize support for Washington State’s Department of Health (WA DOH) and the 27 cases of Myocarditis/Pericarditis identified as potentially associated with COVID-19 mRNA vaccination. STLT responded to WA’s request for assistance and held two calls this week, including with Vaccine TF, to plan an epidemiologic investigation of these cases. WA plans to start collecting medical records in a standardized format as early as 5/28 and will have a CDC epidemiologist deployed to WA to assist.
- The STLT School Field Work Section is providing technical assistance to the Lake County Health Department (Illinois) in evaluating the impact of modified quarantine on secondary transmission of SARS-CoV-2 in 33 K-12 schools. Individuals within 3-6 ft. of a COVID-19 case can be exempted from quarantine and continue in-person learning if mitigation strategies were in place at time of exposure. Exempted contacts get tested 5- 7 days following exposure. Data from these schools will be compared with data on cases and contacts from 15 K-12 schools who will not implement modified quarantine.” [Emphasis added.]
- Extensive emailing occurred about the EOC [Emergency Operations Center] All-Hands Meeting, the largest ever at 2000 attendees [p. 127], followed by many fully redacted pages.
- Many of the full-page redactions are related to the “deliberative process” exemption (b5), but b6, personal privacy, is used as well.
- There are many pages of highly redacted emails involving Rebecca Greco Krone, the CDC Co-Lead, Vaccine Task Force, COVID-19 Response.
23-00756 CDC Records for Requester




My mother and I did not get the vaccine; however, my sister did. She needed eye surgery and the hospital would not do it without the vaccine. She got the first shot on 7/23/21 and the second dose 8/13/2021. I have her vaccination record card. She is now dead. She had a stroke (a huge brain bled) and had a blood clot in her leg. She lived two weeks with no improvements. She died 9/30/2022 appproximately one year after the vaccine. IF they had immediately stopped the vaccines AFTER this report came out, she would not have gotten the shots. This report reached authorities 5/27/2021? If authorities had immediately stopped all vaccines, my sister would have never taken it. As I said, she took the first shot 7/23/2021. So, two months after this report, they were still mandating the vaccine and my sister was forced to take it to get the surgery she needed!!! PEOPLE need to be held accountable. OK. So the vaccines are experimental and therefore protected from lawsuites. BUT once the data was brought forward, those people who ignored the data, hid the data and lied about the data should not be protected by the law. They should be held liable for her death. Her name: Lisa Ann Richards. I consider this murder.