More Horrors, This Time From Moderna – Defending the Republic Lawsuit Obtains Almost 15,000 Pages of Moderna COVID-19 Vaccine Clinical Trial Documents
Defending the Republic on Its Historic Win to Require the FDA to Release Moderna’s Spikevax Clinical Trial Documentation
“As a result of Defending the Republic’s (DTR) successful Freedom of Information Act (FOIA) litigation against the Food and Drug Administration (FDA), we are excited to announce that we are releasing nearly 15,000 pages of documents relating to testing and adverse events associated with Moderna’s COVID-19 vaccine ‘Spikevax.’
DTR filed its FOIA lawsuit after the FDA denied the expedited production of Moderna COVID-19 records, stating there was no compelling need or urgency for the public to review this information. This spring, DTR reached an agreement with the FDA for the production of approximately 24,000 pages of some of the most important records submitted by Moderna in support of its Biologics License Application (BLA). This is the first part of that production. Later this year the FDA will produce approximately 8,000 more pages of Moderna documents.
These documents are the first significant release of data from Moderna’s COVID-19 clinical trials. They reveal the causes of deaths, serious adverse events, and instances of neurological disorders (such as Bell’s Palsy and Shingles) potentially associated with Moderna’s COVID-19 vaccine.
Importantly, these records also demonstrate the utter lack of thoroughness of these studies. Many of those who died after receiving the Moderna vaccine were not given an autopsy. According to one study, 16 individuals died after being administered the Moderna vaccine. The study’s authors indicated that out of those 16 deaths, only two autopsies were performed, five of the dead were not autopsied, and the autopsy status of nine of the dead was ‘unknown’.
Here is a sampling straight from the study:
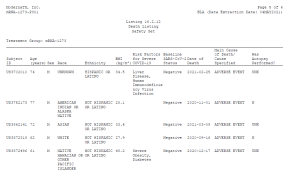
Yet this did not stop those running these ‘studies’ from concluding, despite the absence of evidence, that the Moderna vaccine was not related to these deaths. For example, a 56 year-old woman experienced ‘sudden death’ 182 days after receiving the second Moderna dose. The cause of death was unknown, and no autopsy was conducted. It seems they purposely decided not to investigate suspicious deaths in case the Moderna vaccine might be the cause.
There were numerous examples of participants with post-vaccination Bell’s Palsy and Shingles (Herpes Zoster). One 44 year-old female had ‘left side facial paralysis’ just eight days after the second dose. Numerous vaccinated participants saw the onset of Shingles less than 10 days after vaccination.
Other key observations during the Moderna ‘studies’ involved serious adverse events for those in the vaccinated groups. A number of participants experienced: myocardial infarction (heart attack); pulmonary embolism; spontaneous abortion/miscarriage; transient ischemic attack (TIA); and lymphoma. Subsequent analyses of reports from the FDA VAERS database, the Department of Defense’s DMED database, and European regulators showed heightened rates of these illnesses following administration of the Moderna vaccine.
And similar to their treatment of deaths post-vaccination, the studies seemed predestined to conclude that these serious adverse events – many of them life-threatening – were not related to the Moderna vaccine. It didn’t matter whether the adverse event occurred within days of vaccination. This creates serious doubt concerning the safety of the Moderna vaccine and the FDA’s standards and approval of the Moderna vaccine.
As part of the FDA’s production, DTR also received the results of a study entitled ‘A GLP Intramuscular Combined Developmental and Perinatal/Postnatal Reproductive Toxicity Study of mRNA-1273 in Rats.’ The objective of this study was to: ‘assess the potential effects of mRNA-1273, a vaccine development candidate against SARS-CoV-2 infection, when administered intramuscularly on Study Days 1 and 15 (28 and 14 days prior to mating, respectively) and Gestation Days 1 and 13 on fertility and pre and postnatal development in the pregnant and lactating female Sprague Dawley CD (Crl:CD[SD]) rat.’
The findings of this study are troubling: the mRNA vaccine altered the skeletal variations of the rat fetuses and the ‘female pregnancy index’ of the vaccinated rats was significantly lower than the control group. (A summary of this study was previously reported; DTR, via its FOIA lawsuit, is providing the full study.)
Here are the study’s key findings and observations:
- ‘mRNA-1273-related common skeletal variations consisting of wavy ribs and increase nodules were observed. Wavy ribs appeared in 6 fetuses in 4 litters for a fetal prevalence of 4.03% and a litter prevalence of 18.2%. Rib nodules appeared in 5 of those 6 fetuses. The fetal and litter incidence of wavy ribs exceeded the range observed historically at the Testing Facility (see Appendix 40, Historical Control Data) and the fetal and litter incidence of rib nodules was within the range.’
- ‘mRNA-1273-related, non-adverse effects were limited to increase in the number of fetuses with common skeletal variations of 1 or more rib nodules and 1 or more wavy ribs.’
- ‘The mean number of [reproductive] cycle lengths was statistically-significantly higher in the mRNA-1273 group as compared to the control group.’
- ‘Mating occurred in 95.5% of the rats in the control group and 88.6% of the rats in the mRNA-1273 group.’
- ‘The female pregnancy index (number of rats mating/number of rats in the group) was 93.2% and 84.1% in the control and mRNA-1273 groups, respectively.’
- ‘At scheduled euthanasia, 1 pup in the mRNA-1273 dose group was observed with bilateral, small, minimal renal papilla and another pup from the same litter was observed with left, small, moderate renal papilla. These findings were not considered related to mRNA-1273 because the observations occurred only in 2 pups from a single litter.’
Finally, the FOIA production included a 2017-2018 Moderna study (which was submitted as part of the Spikevax BLA) entitled ‘A Single Dose Intramuscular Injection Tissue Distribution Study of mRNA-1647 in Male Sprague-Dawley Rats.’ (The mRNA-1647 vaccine was developed before Moderna’s COVID-19 vaccine.) The purpose of this study was to ‘to determine the tissue distribution of mRNA-1647, when given once by intramuscular injection to rats’ and to determine the toxicokinetic characteristics of mRNA-1647.’
Testing revealed that ‘mRNA-1647 was detected in all of the analyzed tissues except for kidney’ with elevated levels of mRNA-1647 found in the spleen and eye. Notably, mRNA-1647 was detected in the brain and heart (see charts below).
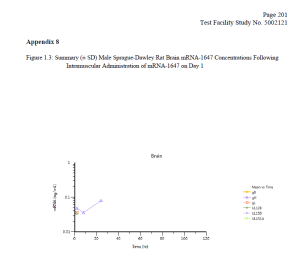
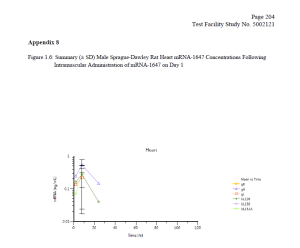
DTR understands the necessity for the public review of the Moderna files and is making them available for download below. Please consider supporting our efforts to expose government corruption, promote government accountability and transparency, fight COVID-19 mandates on behalf of military servicemembers [sic], combat censorship, and to restore integrity in our elections.”
MODERNA SUMMARY OF FINDINGS
(Based on documents below, please see individual documents and search each for more information)
Moderna Clinical Study Report 16.2.7 Adverse Event Listing (May 2021) (11,505 pages)
Moderna Clinical Study Report 16.2.7 Adverse Event Listing (May 2021) (218 pages)
Moderna Clinical Study Report 16.2.7 Adverse Event Listing (Nov. 2020) (1,650 pages)
Moderna Clinical Study Report 16.2.7 Adverse Event Listing (June 2021) (312 pages)



