Report 38: Women Have Two and a Half Times Higher Risk of Adverse Events Than Men. Risk to Female Reproductive Functions Is Higher Still.
The Pfizer documents demonstrate a strong signal that women have far more adverse events than males, particularly when considering reproductive organs and function. Primary source material from Pfizer shows a strong, sex-linked Adverse Event (AE) difference. Two major data collections, Reissue of Pfizer’s “5.3.6 Cumulative Analysis of Post-Authorization Adverse Event Reports of PF-07302048 (BNT162B2) Received Through 28-FEB-2021” and “APPENDIX 2.1 Cumulative Number of Case Reports (Serious and Non-Serious, Medically Confirmed and Non Medically-Confirmed) from Post-Marketing Data Sources, Overall, by Sex, Country, Age Groups and in Special Populations and Summary Tabulation by Preferred Term and MedDRA System Organ Class,” show substantially greater numbers of Adverse Events in women contrasted with men. This signal is particularly strong for the reproductive organs and their functions. Women have approximately three times the risk of Adverse Events than do males, and the specific risk to the reproductive organs and their functions is even stronger.
Two large data sets in the Pfizer confidential document collection, released pursuant to a court order, report consistent sex differences in the absolute number and percentage of Adverse Events (AEs) and Adverse Events of Special Interest (AESI). This report will examine primary source documents that collect Adverse Events at two points in time – February 28, 2021, the end of first two and a half months following widespread inoculation with Pfizer’s COVID-19 vaccine, and then at a second time ending on March 15, 2022.
Most AEs appear to have been spontaneously reported through a mechanism the public is still waiting to learn about, which means they were not part of a well-regulated and proactive surveillance program and may underestimate the actual frequency of such events.
Many people having a complication related to Pfizer’s Lipid Nanoparticle Messenger Ribonucleic Acid (LNP/mRNA) prodrug, BNT162b2 (the Pfizer COVID-19 vaccine), are not aware of how to report or are unable to report in cases of a severe complication. Alternatively, reporting may be being actively suppressed.
As a review of the entries in Appendix 2.1, the 170-page registry of 4,563,770 Adverse Events logged in by April 15, 2022, shows that over-reporting and, in some cases, questionable relevance of the reporting in some disease categories is a possibility.
Sex Differences Example 1:
Reissue of Pfizer’s 5.3.6 Cumulative Analysis of Post-Authorization Adverse Event Reports of PF-07302048 (BNT162B2) Received Through 28-FEB-2021
The FDA reissued Pfizer’s 5.3.6 Adverse Events document on April 1, 2022, and it offers a summary of Adverse Events and Adverse Events of Special Interest after injection of BNT162b2, Pfizer’s LNP/mRNA vaccine.
This data set comprises 42,086 subjects from the first two and a half months following the Emergency Use Authorization (EUA) issued by the Food and Drug Administration (FDA) on December 11, 2020.
Table 1 below shows a tally of Adverse Events and Adverse Events of Special Interest by organ system from the 5.3.6 Reissue document, although it must be pointed out that some cases were reassigned to organ categories by the author.
For instance, myopericarditis was moved from Pfizer’s Autoimmunity assignment to Cardiac based on the organ involved rather than the assumed disease process.
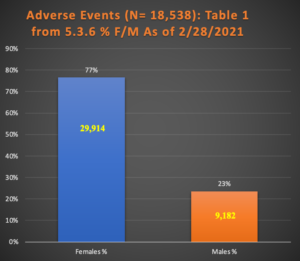
Table 1: AEs and ASEIs up to 2/28/2021
In every category, females substantially outnumber males. Charts 1 and 2 are graphical representations of this data.
| Study | Females % | Males % | F | M | N = | Unk | p |
| Table 1 from 5.3.6 | 77% | 23% | 29914 | 9182 | 42086 | 2990 | p < 0.001 |
| Table 7 from 5.3.6 | |||||||
| Autoimmune | 81% | 19% | 682 | 156 | 838 | N/A | p < 0.001 |
| Cardiac | 77% | 21% | 1076 | 291 | 1403 | 36 | p < 0.04 |
| Covid-19 | 66% | 34% | 1650 | 844 | 3067 | 573 | p < 0.001 |
| Dermatologic | 94% | 6% | 17 | 1 | 19 | 1 | See note
below Chart 1 |
| Hematologic | 75% | 25% | 676 | 222 | 898 | 0 | p = 0.385 |
| Hepatic | 61% | 37% | 43 | 26 | 70 | 0 | p =0.019 |
| Musculoskeletal | 80% | 20% | 2760 | 711 | 3471 | 0 | p < 0.001 |
| Neurologic | 69% | 31% | 623 | 283 | 927 | 21 | p < 0.001 |
| Other (Pyrexia and Herpes) | 76% | 24% | 5969 | 1860 | 7829 | 0 | p = 0.527 |
| Renal | 67% | 33% | 46 | 23 | 69 | 0 | p = 0.085 |
| Respiratory | 55% | 45% | 72 | 58 | 130 | 0 | p < 0.001 |
| Stroke | 67% | 33% | 182 | 91 | 273 | 0 | p = 0.001 |
| Thromboembolic event | 62% | 38% | 89 | 55 | 144 | 0 | p < 0.001 |
| Vasculitis | 81% | 19% | 26 | 6 | 32 | 0 | p = 0.549 |
| Total excl. Unknown | 75% | 25% | 13911 | 4627 | 18538 |
Chart 1 illustrates this finding with 29,914 females with AEs compared with only 9,182 for males. (i.e., p < 0.001).
It should be noted that “p,” as shown in p < 0.001 above, indicates the level of significance. Commonly, p < 0.05 is the minimal level of acceptance, meaning there is a 95% chance that the number is the true number with a certain confidence interval. Therefore, p < 0.001 indicates a 99.999% probability that the number did not occur by chance. “p” values this low are rarely seen in clinical medical studies.
Chart 1: Female/Male Ratio in 39,096 Subjects
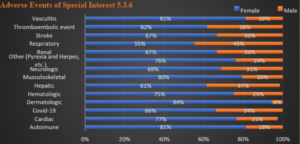
This trend follows through Table 7 (AESI), from 5.3.6 Reissue. Chart 2 shows the female-to-male ratio as percentages for each organ system as reported. Note that females substantially outnumber males in all categories and by more than a factor of three overall.
There is no category in which the number of cases for males outnumber females. Statistical significance exists at p < 0.05 in comparison of the rates of particular types of AEs in females versus males. Hematologic, Dermatologic, Other (Pyrexia and Herpes), Renal and Vasculitis all appear as exceptions with p values > 0.05. Note: Dermatologic was evaluated using Fisher exact test due to small sample size, p = 0.093.
Chart 2: Organ System Detail
Sex Differences Example 2: Appendix 2.1
A second large series of Adverse Events associated with Pfizer’s BNT162b2 vaccine document trove, Appendix 2.1, recently surfaced following a FOIA request from the Australian Therapeutic Goods Administration (TGA) and consists of a 170-page document that tallies Adverse Events by diagnosis in 1,348,079 subjects (i.e., patients). The sex was known in 1,282,113 cases – 923,194 women (72% of those with known sex and 68% of total series including unknown sex) and 358,919 men. Data capture ended on April 15, 2022.
The total number of Adverse Events reported in this document is 4,563,770 for an average of 3.4 AEs per case. The disproportionate representation of AEs in females presents again strongly here, as it did in Pfizer’s 5.3.6 Reissue document.
Table 2: Female:Male Difference in 1,282,113 Cases of Adverse Events
| Study | Females % | Males % | Females | Males | N = | |
| Appendix 2.1
16-April-2022 |
72% | 28% | 923194 | 358919 | 1282113 | |
Chart 3: Female:Male Comparison in AEs Subjects
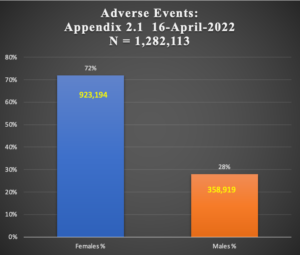
Adverse Events occur two and a half times more in women than men as shown in Chart 3 above. This is the same pattern seen in the earlier reporting of a smaller series from Document 5.3.6, p < 0.001.
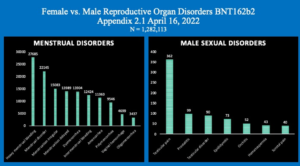
Chart 4 illustrates this same disparity in the specific data referable to female and male reproductive organ and organ function disorders with much higher absolute numbers for women as well as in terms of percent of adverse events.
A striking difference is shown here with 148,874 women reporting Reproductive System AEs contrasted with only 1,745 males, p < 0.001.
Chart 4: Reproductive Organ and Function Sex Differences

As seen in Chart 5, below left, females appear to have fewer diagnostic categories than males but only because there are so many for women that a charting of them is too busy if all are plotted.
For comparison of the sexes see Appendix 2 (females) and Appendix 3 (males) that list the reported reproductive organ and organ function disorders by sex following injection of Pfizer’s BNT162b2. This tally lists diagnoses with reporting frequency of ten or more.
Chart 5 shows the numbers of the just the top ten menstrual dysfunctions contrasted with the much smaller number of reproductive issues in men.
Chart 5: Menstrual Disorders compared with Male Reproductive Disorders
Why do Women Have So Many More Adverse Events than Males?
No immediate answer to this question exists. However, the signal is strong.
Is there some distortion in the reporting mechanism that might explain such a wide difference? Perhaps. Is there some kind of systematic reporting bias? We can only speculate at present.
Alternatively, are there true sex differences in reaction to Pfizer’s LNP/mRNA injections? Are women more prone to having complications after receiving Pfizer’s BNT162b2 vaccine? Perhaps. Is there something about the LNP/mRNA concentration in ovaries that leads to production of more mRNA transcribed Spike or Spike-related proteins that have been shown to be toxic in multiple studies.
We have seen from the preclinical animal studies, Chart 6 following, that ovaries are one of the top four organs as far as concentration of LNP/mRNA is concerned. But, unfortunately, this study in Wistar Han Rats only ran for two days and no longer-term studies were performed. Furthermore, the ovaries – like liver, spleen and adrenal glands – had LNP/mRNA concentrations that were steeply rising at the time of animal sacrifice.
Had autopsies had been performed in a systematic manner following widespread human inoculation in individuals dying in the weeks following injection of Pfizer’s BNT162b2, we may have had the answer by now and would certainly know more about gross and microscopic changes occurring in organs following the injection. Spike and related protein levels in the various organ systems would be of great interest.
Chart 6 illustrates deposition of LNP/mRNA at the injection site, left chart, followed by rapid dissemination throughout the body with concentration in four organs, liver, spleen, adrenal glands and ovaries, right chart.
Chart 6: Distribution of LNP/mRNA in Wistar Han Rats
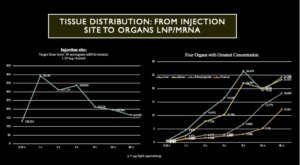
LNP/mRNA concentrates in ovaries as shown in Chart 6 illustrating data from preclinical studies performed in Wistar Han Rats. Note: The X-axis is nonlinear in Charts 6 and 7. Interpret the data accordingly.
Caution is needed here as animal studies may be misleading. There is such a thing as species-specific reactions, and humans may have different findings.
Chart 7 illustrates the disparity between ovaries and testes with respect to LNP/BNT162b2 uptake showing more than 38 times more concentration in ovaries than testes, as shown in these animal studies.
Chart 7: Tissue Concentration of LNP/mRNA Ovaries vs. Testes
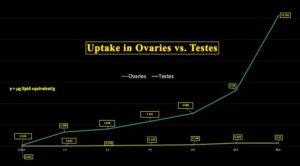
Why do ovaries concentrate lipid nanoparticles and mRNA contained therein so much more effectively than testes?
And does this account for the large disparity in the incidence of Adverse Events and Adverse Events of Special Interest following injection of BNT162b2 in women as opposed to men?
Or are these differences in AEs overall and with respect to the dysfunction in the Reproductive Systems specifically a result of some methodological quirk?
We cannot definitively answer that question at present. For now, we must interpret these data as showing women are at increased risk for Adverse Events from Pfizer’s LNP/mRNA product than are men, both in terms of many or all organ systems but especially with respect to reproductive organ systems and their functions.
Assuming this differential is caused by the disproportionate impact of BNT162b2 on women and their reproductive systems and organs, the implications could be profound.
Appendix 1: Female Reproductive AEs Following Inoculation with BNT162b2
148,874 reproductive organ AEs occurred in women which represents ~16% of the total number of Adverse Events in women. The list below gives the diagnoses reported 10 or more times.
| Total AEs N = | 923194 |
| Heavy menstrual bleeding | 27685 |
| Menstrual disorder | 22145 |
| Menstruation irregular | 15083 |
| Menstruation delayed | 13989 |
| Dysmenorrhea | 13904 |
| Intermenstrual bleeding | 12424 |
| Amenorrhea | 11363 |
| Polymenorrhea | 9546 |
| Breast pain | 4800 |
| Vaginal hemorrhage | 4699 |
| Oligomenorrhea | 3437 |
| Hypomenorrhea | 2643 |
| Postmenopausal hemorrhage | 2456 |
| Abortion spontaneous | 1809 |
| Breast swelling | 1339 |
| Menstrual discomfort | 1199 |
| Premenstrual syndrome | 998 |
| Breast tenderness | 792 |
| Menometrorrhagia | 632 |
| Adnexa uteri pain | 609 |
| Premenstrual pain | 585 |
| Breast enlargement | 483 |
| Vaginal discharge | 480 |
| Breast discomfort | 443 |
| Mastitis | 392 |
| Ovulation pain | 347 |
| Endometriosis | 337 |
| Menstrual cycle management | 308 |
| Anovulatory cycle | 273 |
| Uterine pain | 270 |
| Abnormal withdrawal bleeding | 265 |
| Uterine hemorrhage | 231 |
| Vulvovaginal pain | 191 |
| Ovulation delayed | 181 |
| Premature baby | 181 |
| Vulvovaginal mycotic infection | 173 |
| Breast cancer | 147 |
| Fetal death | 147 |
| Fetal growth restriction | 124 |
| Vulvovaginal candidiasis | 122 |
| Breast cyst | 115 |
| Genital hemorrhage | 115 |
| Breast edema | 113 |
| Abnormal uterine bleeding | 100 |
| Pelvic venous thrombosis | 98 |
| Labor pain | 95 |
| Uterine leiomyoma | 91 |
| Polycystic ovaries | 82 |
| Breast discharge | 71 |
| Vulvovaginal pruritus | 71 |
| Breast disorder | 68 |
| Uterine contracture during pregnancy | 68 |
| Ectopic pregnancy | 67 |
| Premature labor | 64 |
| Morning sickness | 62 |
| Vaginal infection | 60 |
| Vulvovaginal discomfort | 59 |
| Abortion | 58 |
| Premature menopause | 58 |
| Vulval ulceration | 56 |
| Stillbirth | 56 |
| Vulvovaginal dryness | 54 |
| Coital bleeding | 46 |
| Ovarian cyst rupture | 44 |
| Premature delivery | 44 |
| Endometrial thickening | 42 |
| Genital burning syndrome | 42 |
| Adenomyosis | 41 |
| Breast abscess | 41 |
| Fetal heart rate abnormal | 41 |
| Menarche | 40 |
| Premenstrual headache | 40 |
| Uterine contractions abnormal | 40 |
| Breast induration | 39 |
| Premature rupture of membranes | 37 |
| Uterine polyp | 37 |
| Vulvovaginal swelling | 37 |
| Abortion induced | 36 |
| Uterine inflammation | 36 |
| Vulval hemorrhage | 34 |
| Pelvic inflammatory disease | 33 |
| Pregnancy | 32 |
| Pelvic discomfort | 30 |
| Premature menarche | 27 |
| Premature ovulation | 27 |
| Breast hematoma | 26 |
| Infertility female | 26 |
| Postpartum hemorrhage | 26 |
| Uterine disorder | 26 |
| Pelvic hemorrhage | 25 |
| Noninfective oophoritis | 23 |
| Vaginal ulceration | 23 |
| Dyspareunia | 22 |
| Ovarian disorder | 22 |
| Unintended pregnancy | 22 |
| Vaginal order | 22 |
| Vulvovaginal inflammation | 21 |
| Breast cancer | 20 |
| Breast disorder female | 20 |
| Hemorrhagic ovarian cyst | 20 |
| Placental disorder | 20 |
| Gestational diabetes | 19 |
| Abortion early | 19 |
| Endometrial disorder | 18 |
| Nipple inflammation | 18 |
| Endometrial hyperplasia | 18 |
| Ovarian hemorrhage | 17 |
| Ovarian failure | 16 |
| Vulvovaginal erythema | 16 |
| Ovarian vein thrombosis | 15 |
| Polymenorrhagia | 15 |
| Threatened labor | 14 |
| Fibrocystic breast disease | 13 |
| Ovarian enlargement | 13 |
| Uterine enlargement | 13 |
| Cervix hemorrhage uterine | 12 |
| Breast atrophy | 11 |
| Breast hemorrhage | 11 |
| Breast neoplasm | 11 |
| Cesarean section | 11 |
| Cervical dysplasia | 11 |
| Pelvic girdle pain | 11 |
| Vaginal disorder | 11 |
| Vulval disorder | 11 |
| Bartholin’s cyst | 10 |
| Decidual cyst | 10 |
| Fetal cardiac disorder | 10 |
| Fetal growth abnormality | 10 |
| Fetal vascular malperfusion | 10 |
| Vaginal cyst | 10 |
| Small for dates baby | 10 |
| Vaginal cyst | 10 |
Appendix 2: Male Reproductive Disorders Following Inoculation with BNT162b2
1,745 reproductive organ AEs were reported in men which represents 0.49% of the total number of Adverse Events in men. AEs list occurred 10 or more times.
| Males | |
| Total AEs = | 358919 |
| Testicular pain | 362 |
| Prostatitis | 99 |
| Testicular disorder | 90 |
| Epididymitis | 73 |
| Orchitis | 52 |
| Hematospermia | 43 |
| Scrotal pain | 40 |
| Penile pain | 31 |
| Penis disorder | 31 |
| Benign prostatic hypertrophy | 26 |
| Penile swelling | 25 |
| Scrotal swelling | 24 |
| Erection increased | 23 |
| Testicular disorder | 22 |
| Orchitis noninfective | 20 |
| Ejaculation disorder | 18 |
| Ejaculation failure | 18 |
| Prostatomegaly | 18 |
| Priapism | 17 |
| Testes discomfort | 16 |
| Spontaneous penile erection | 15 |
| Penile edema | 13 |
| Prostatic disorder | 13 |
| Penile hemorrhage | 11 |
| Penile erythema | 10 |
| Penile vein thrombosis | 10 |
| Scrotal erythema | 10 |
Author: Robert W. Chandler, MD, MBA, Team 5




Work At Home I even have created 96,760 Buck simply last month by operating Aia1 on-line from my home. (C110 i’m a full time college man and just doing this in my free time for few hours per week by mistreatment my laptop computer A.
Everyone will check this out …… http://holdonwork24.blogspot.com
https://r.search.yahoo.com/_ylt=AwrkORY1NSFkPuwVdjC0eSI5;_ylu=Y29sbwMEcG9zAzgEdnRpZAMEc2VjA3Ny/RV=2/RE=1679926709/RO=10/RU=https%3a%2f%2fwww.huffpost.com%2fentry%2fbill-gates-coronavirus-vaccine-conspiracy_n_5eb9ab7ac5b69358ef8a9803/RK=2/RS=nHSHppkgs4XdPgw9WlqebplbX4g-
I am 80 years old in October and I have always believed in the saying,
“IF IT LOOKS LIKE A DUCK, AND QUACKS LIKE A DUCK, THEN IT IS A DUCK”!
https://r.search.yahoo.com/_ylt=AwrkORY1NSFkPuwVcTC0eSI5;_ylu=Y29sbwMEcG9zAzMEdnRpZAMEc2VjA3Ny/RV=2/RE=1679926709/RO=10/RU=https%3a%2f%2fwww.ecdc.europa.eu%2fen%2fcovid-19%2fdata/RK=2/RS=dAO8EEp8RCCDLcaDwgwVe25Osdc-
“IF IT LOOKS LIKE A DUCK, AMD QUACKS LIKE A DUCK, THEN IT IS A DUCK”!
I’ve never left a comment before so I want to make sure that anything I comment on WILL NOT BE FOR PUBLIC VIEWING!!! I’ve been an avid follower of Dr Wolf long before the Pfizer document’s release and the Pfizer book which I bought and love! Thank to ALL for your hard work!
Do you have any info on the website blessedbyhisblood.com? Having access to unvaccinated blood is very important to me and my family! I was thinking about joining this website but was not sure. Any suggestions?
99.9%, not 99.999%.