Report 07: COVID-19 Vaccines and Pregnancy: Risky Business
COVID-19 Vaccines & Pregnancy: Risky Business
This report has been brought to you by DailyClout
To date there have not been any human clinical trials conducted by a COVID-19 vaccine pharmaceutical company to determine if vaccines are safe during pregnancy or while breastfeeding. All Emergency Use Authorizations (EUA’s) exclude pregnant women and no COVID-19 vaccine has been approved for use during pregnancy. Astonishingly, however, many professional medical organizations have strongly advocated for their use during pregnancy despite the lack of any safety data.
Unfortunately, as more pregnant women have been vaccinated, serious adverse events are being exposed in both Pfizer documents and in the Department of Defense (DOD) medical database.
Thanks to a court ordered release of confidential Pfizer documents (the FDA wanted these documents sealed for 75 years) we have learned that pregnant women and breastfeeding mothers were excluded from phase 1, 2 and 3 of the human trials. One recently released Pfizer document lists 21 groups of people who were excluded from all phases of the Pfizer trials and specifically singles out “women who are pregnant or breastfeeding” as not able to participate in any of the trials https://www.phmpt.org/wp-content/uploads/2022/04/125742_S1_M5_5351_c4591001-fa-interim-sample-crf.pdf (Annotated Study Book for Study Design: C4591001 Study Design Version: 11.0, 2020, Page 33 item 2.h 11, exclusion 11A00 under exclusion criteria).
Despite this, organizations such as the American College of Obstetrics and Gynecology (ACOG) and The Society for Maternal-Fetal Medicine (SMFM) are strong advocates for vaccinating pregnant and lactating women. In an unprecedented manner, ACOG persistently advocated for pregnant women to get vaccinated while acknowledging in their clinical guidelines that “none of the COVID-19 vaccines approved under EUA have been tested in pregnant individuals.” https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care So how could they possibly be promoting an experimental and untested vaccine for pregnant women? As it turns out their clinical recommendations are based on a faulty study conducted on a few dozen rats in France.
Before any research trials can be performed on human pregnant women, a new drug must first be tested on pregnant animals. These are called Developmental and Reproductive Toxicity or (DART) studies. In ACOG’s clinical guidelines, they stated that the “DART studies for the Pfizer-BioNTech COVID-19 vaccine have been reported in Europe… According to the report animal studies using the Pfizer/BioNTech COVID-19 vaccine do not indicate direct or indirect harmful effects with respect to pregnancy, embryo/fetal development, parturition, or postnatal development.” https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care
So we see that their confidence in the safety of the Pfizer vaccine is based solely on animal studies. Given the extreme importance of studying the effects of a new vaccine technology on pregnant women and their offspring; one would expect this study to be conducted by independent researchers using a robust design that answers fundamental questions. Questions like were there any congenital abnormalities or developmental issues in the offspring and were there any long-term effects on fertility?
After a review of this study, it is astounding to discover that it was performed on a mere 44 rats and for a length of only 42 days! https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8163337/ To their credit it turns out that rats are the perfect mammal to do pregnancy studies on because they only need 21 days from conception to delivery. Half of the rodent pregnancies were terminated at day 21 via cesarean section and the fetuses were removed from the mother. All were euthanized and then anatomically studied. The other half were allowed to deliver naturally and then were monitored until they were weened at 21 days of age when the rest were euthanized. This is long before any developmental issues could have been observed in the offspring and precludes any long-term safety or fertility studies of the mothers or their offspring. The effects on fertility in this study were determined by dissection and examination of the ovaries of the mother rats who were fully mature at the time of vaccination.
After this 42-day study on 44 pregnant rats they concluded that there were “no effects on female fertility and prenatal and postnatal offspring development in rats with BNT162b2, mRNA-based COVID-19 vaccine.” Thus, supposedly, the prerequisite for a DART study was complete. However, there are at least two glaring problems with this study.
First, it does not fulfill the requirements of a DART study, which is “to detect any effects of a drug within a complete reproductive cycle as relevant to humans: from initial conception to reproductive capacity in the next generation.” There is no way to know if any adverse effects on the development of those newborn rats occurred, let alone to know if their reproductive capacity (fertility) was altered.
Second, there was a significant conflict of interest with the studies’ investigators. The “Declaration of Competing Interest” disclaimer at the bottom of the publication reveals that nine out of ten of the authors of the study were employed by and held stock in either Pfizer or BioNTech. There is no way these investigators could be unbiased; they all had a vested interest in a positive outcome for vaccine trials to move forward. Any negative result would have put a complete halt to any human clinical trial. It would seem they hid this fact as best they could. These are the authors listed at the top of the article: Christopher J. Bowman, Marie Bouressam, Sarah N. Campion, Gregg D. Cappon, Natasha R. Catlin, Mark W. Cutler, Jan Diekmann, Cynthia M. Rohde, Rani S. Sellers, and Claudia Lindemannd.
There is a disclaimer listed at the very bottom on the last page of the article. It only uses initials, so it is easy to miss. Compare the initials from the disclaimer at the very end to the authors listed at the beginning.
Despite this, pregnant women in the United States were encouraged to get vaccinated based on an extremely limited DART animal study that had obvious conflicts of interest. These women, likely out of fear of COVID-19 and with the reassurance of the CDC, FDA, and medical professional organizations, received the vaccine. By the end of 2020 and into 2021, thousands of pregnant women received vaccinations during pregnancy with no EUA approval.
It is notable that even with all the organizations promoting vaccination during pregnancy, the World Health Organization recommended against it until at least January of 2021. Now they don’t recommend against it but instead recommend that pregnant women should weigh the potential risks against the benefits, while simultaneously admitting that there is no long-term safety data available. Either way, since the vaccines have been broadly deployed a great deal of data has been compiled.
So, what does the “safety data” that has been collected on mRNA COVID-19 vaccinated pregnant women show? The FDA requires Pfizer to collect any publicly available data on adverse events related to vaccination once it goes to market. Confidential document (5.3.6 Cumulative Analysis of Post-authorization Adverse Event Reports) contains case reports for the first 68 days of vaccine rollout (from 12/20/2020 to 2/28/2021). The section covering pregnancy and lactation on pages 12-13, reveals that 20% of the 413 reported cases of adverse events were “serious.” These included 25 miscarriages, 5 fetal deaths as well as uterine contractions during pregnancy, preterm deliveries, premature rupture of membranes and fetal growth restriction. Also included were serious and less serious adverse side effects of breast-fed babies that included infantile vomiting, fever, rash, agitation, and allergy to the vaccine. There were also 6 cases of women having adverse events who received COVID-19 vaccine while breast feeding; some of these include paresis (partial paralysis), suppressed lactation, breast pain, migraines and breast milk discoloration. Pfizer’s response to the above alarming data was, “There were no safety signals that emerge from the review of these cases of use in pregnancy and while breast feeding.”
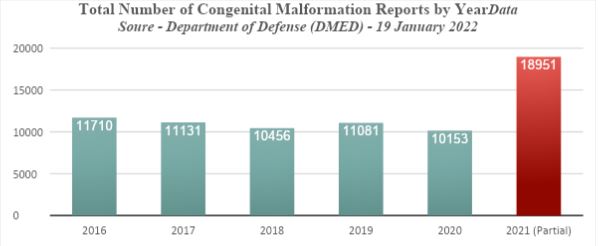
Probably the largest and most reliable health database on overwhelmingly healthy and fit military personnel is collected by the Department of Defense (DoD). This has recently been exposed by three whistleblowers represented by Attorney Thomas Renz. https://www.health.mil/Military-Health-Topics/Health-Readiness/AFHSD/Data-Management-and-Technical-Support/Defense-Medical-Epidemiology-Database They observed disturbing evidence of dramatic increases in serious medical conditions among military personnel in 2021, correlating directly with the roll out of COVID-19 vaccines. Among the numerous conditions listed are congenital malformations.
The rise in congenital malformations increased dramatically from a baseline rate of 10,906 cases per year, to 18,951 for only part of the year in 2021.

Having shown that there is significant risk involved in taking the vaccine when pregnant, let’s now consider the supposed benefits touted by the NIH, CDC and others.
The NIH says “The COVID-19 Treatment Guidelines Panel recommends against withholding treatment for COVID-19 and SARS-CoV-2 vaccination from pregnant or lactating individuals because of theoretical safety concerns (AIII)” (emphasis added). The (AIII) at the end is important. “A” indicates they strongly recommend this and “III” indicates the lowest available rating for evidence used, which is “Expert opinion.” https://www.covid19treatmentguidelines.nih.gov/special-populations/pregnancy The CDC says, “Limited information suggests that pregnant women with COVID-19 might be at increased risk for severe illness compared with nonpregnant women”. https://www.cdc.gov/mmwr/volumes/69/wr/mm6944e3.htm The word “suggests” has a specific meaning in this statement: “The word “suggested” is used when the strength and direction of the results are unified, but results do not achieve statistical significance.” https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/systematic-review-process.html
In layman’s terms the NIH is saying that they strongly suggest that pregnant women be vaccinated for COVID-19 based upon the recommendation of “expert opinion” from groups such as ACOG and SMFM alone, not based on any reliable evidence from one or more randomized trials without major limitations. And we know that ACOG’s “expert opinion” relied heavily upon the limited Pfizer-BioNTech DART study. The CDC is acknowledging that there is limited information supporting the claim that pregnant women with COVID-19 might be at increased risk for severe disease compared with non-pregnant women because the study results claiming this risk cannot prove statistical significance to back up that claim.
The evidence is clear that the potential risks of pregnant women getting vaccinated with the new mRNA COVID-19 vaccines far outweigh the touted yet unproven benefits. The alarming safety signals revealed in the Pfizer documents and DOD database along with the lack of any long term safety data overwhelmingly leads to the conclusion that getting vaccinated during pregnancy is a Risky Business… Risky for the people getting vaccinated and big Business for the pharmaceutical industry.




