Report 81: Summary of 2.4 Nonclinical Overview – Pfizer mRNA COVID-19 Vaccine, BNT162b2
Reference document Pfizer confidential document “2.4 NONCLINICAL OVERVIEW,” Version 3 (36 pages) while reading this summary. [https://phmpt.org/wp-content/uploads/2022/03/125742_S1_M2_24_nonclinical-overview.pdf]
- Table of Contents pps. 2-3
- Abbreviations pps. 4-5
- Nonclinical Testing Strategy Overview pps 6-9
- Pharmacology pps. 10-11
- Immunogenicity pps. 11-13
- Pharmacokinetics pps. 15-20
- Toxicology pps. 21-29
- Genotoxicity, Carcinogenicity, Reproductive and Developmental Toxicity pps. 29-31
- Integrated Overview and Conclusions pps. 32-33
- References pps. 34-36
- Nonclinical Testing Strategy:
Investigational vaccine contents: Nonclinical studies tested two variants, BNT162b2 V.8 and BNT162b2 V.9, that differ in codon optimization designed to “improve antigen expression.” P6¶2 V9 was the candidate submitted for EUA and was the only version used in clinical trials. S proteins produced by V8 and V9 were reported to be identical. mRNA is formulated in lipid nanoparticles for preservation of mRNA and to facilitate delivery to ACEII cells.
BNT162b2 Contents:
- mRNA expressing full length S protein with two proline mutations to “lock the transmembrane protein in an optimal prefusion conformation.”
- Lipids:
- ALC-0315: [(4-hydroxybutyl) azanediyl]di(hexane-6,1-diyl) bis(2-hexyldecanoate)
- ALC-0159: (2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide)
- DSPC: (1,2-disteroyl-sn-glycero-3-phosphocholine)
- Cholesterol
- Sucrose
- NaCl
- KCl
- Na2HPO4
- KH2PO4
In vitro and in vivo evaluation of primary pharmacology, distribution, metabolism and safety with nonclinical pharmacology, pharmokinetic and toxicity studies in mice, rats and rhesus macaques.
Immunogenicity was evaluated by:
- Serum antibody responses in mice and rats tested by
- S1 and RBD-binding IgG responses by ELISA.
- Functional antibody response by SARS-CoV-2 pseudotype neutralization assay (pVNT).
- Non-human primate (NHP) studies S1 binding IgG responses tested in direct Luminex based immunoassay (dLIA).
- Mouse and non-human primates S-specific T cell responses asses by IFNg ELISpot and intracellular flow cytometry based analysis of the Th1/Th2 profile using splenocytes.
- Challenge study in NHP to assess protection against infection and “…to demonstrate lack of disease enhancement.” P.7 ¶1.
Analysis of LNP
Non-SARS-CoV-2 platform supporting BNT162b2 was demonstrated in LNP-formulated modRNA encoding luciferase testing and the pharmokinetics (PK) of ALC-0315 and ALC-0159 using BALB/c mice and Wistar Han rats.
Metabolism of ALC-0315 and ALC-0159 was studied in mouse, rat and human blood, liver, microsomes, S9 fractions and hepatocytes and in vivo in rat plasma, urine, feces, and liver samples from the PK study.
V8 and V9 Toxicology and DART Study
BNT162b2 V8 and V9 were studied in repeat dose toxicity one study for each variant. A Developmental and Reproductive Toxicology (DART) study was performed on V9 in rats.
The location of records for inspection is included in each final study report.
- Pharmacology:
Mechanism of action: the nucleoside-modified mRNA encodes SARS-CoV-2 full length spike glycoprotein (S). The functional and structural lipids encapsulate the mRNA for preservation and to facilitate entry into the host ACEII cells. Human neutralizing antibodies demonstrate BNT162b2 authentically “…presents the ACE2 binding site and other epitopes targeted by many SARS-CoV-2 neutralizing antibodies.” P 10 ¶1.
Most antibodies with SARS-CoV-2 neutralizing activity are directed against the RBD. “Efficient in vitro expression of the P2 S protein was demonstrated in vitro transfection of cells with BNT162b2 RNA drug substance and BNT162B2 drug product.” P11¶1.
- Immunogenicity of V9 in mice:
- Strong antigen-binding IgG and high titer neutralizing antibody response that recognizes S1 and the RBD and elicited high neutralizing titers in pseudotype neutralization assays.
- Th1-phenotype CD4+ response.
- INFg+, Il-2+ CD8 T-Cell response with a single dose.
- Splenocytes @ 28 days showed robust CD4+ and CD8+ T-Cell IFNg responses that were first identified at day 12.
SARS-CoV-2 Challenge in Rhesus Macaques:
- Covid in Rhesus Macaques: Transient upper and lower airway infection peaking Day 2 to 4 and viral replication in GI tract.
- S1 binding IgG and SARS-CoV-2 neutralizing titers 14 days after single injection with substantial increases after the second injection.
- Strong S-specific Th1-dominant INFg+ T-Cell responses were detected in all immunized rhesus macaques.
- None of the challenged immunized animals showed clinical signs of significant illness, indicating that the 2-4 years old male rhesus challenge model is primarily an infection model not a COVID disease model.” P13¶1
- There was no evidence of vaccine-elicited disease enhancement.
Female Rat DART Study:
- 4 doses IM in female rats 21 and 14 days before mating then on gestational days 9 and 20. Sera was collected at the end of gestation, end of lactation and offspring.
- Neutralizing titers were present in both maternal females and pups. P13¶5.
- Pharmacokinetics: ADME Profile LNP
- ALC-0315 and ALC-0159 injected IM distribute from the plasma to the liver. Urinary excretion was nil and % of dose excreted in feces was ~1% for ALC-0315 and ~50% for ALC-0159.
- Luciferase was detected at the injection site at 6 hours and not detected after 9 days and in liver @ 6 hours and was not detected after 48 hours. P15¶2.
- “The greatest mean concentration of LNP was found remaining in the injection site in both sexes. Total recovery (% of injected dose) of LNP outside the injection site was greatest in the liver and was much less in the spleen, adrenal gland, and ovaries.” P15¶2.
- In vitro metabolism of ALC-0135 and ALC-0159 was evaluated in blood, liver microsomes, S9 fractions and hepatocytes from mice, rats, monkeys, and humans.
- In vivo metabolism was examined in rat plasma, urine, feces and liver samples from the PK study. P15¶3.
- “Metabolism of ALC-0315 and ALC-0159 appears to occur slowly in vitro and in vivo.” P15¶3.
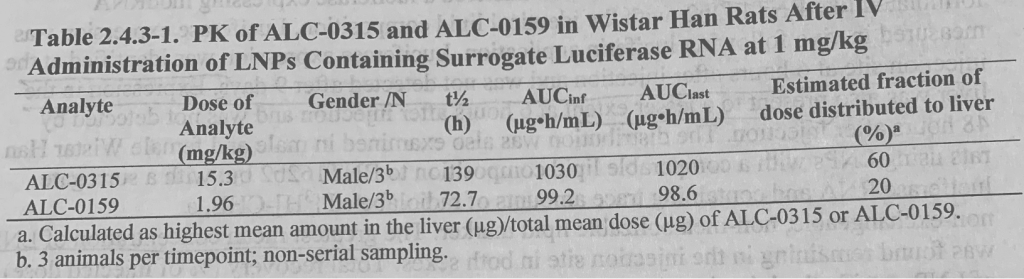
Note the estimated fraction of dose distributed to liver 60% for ALC-0315 and 20% for ALC-0159. P16¶4.
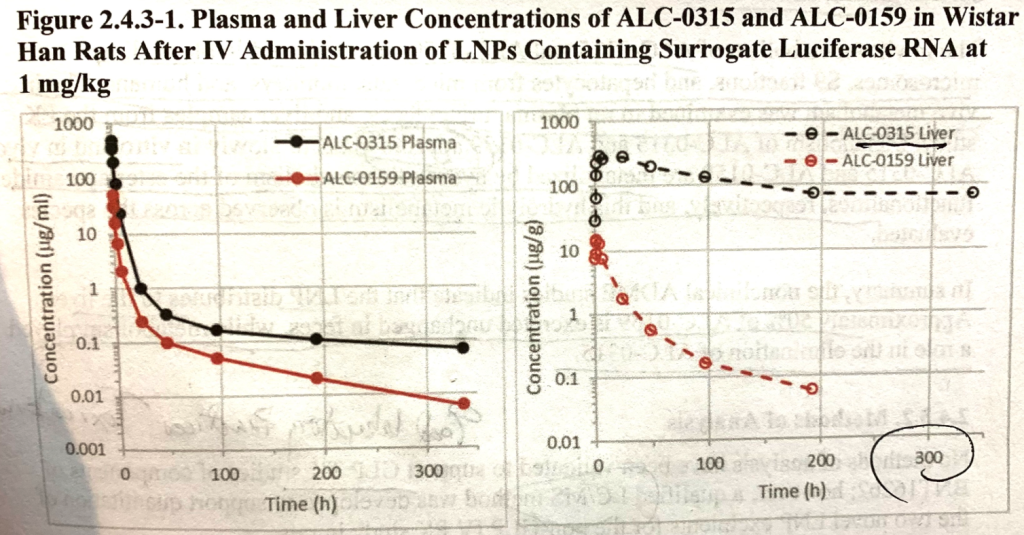
ALC-0315 is retained in the liver. P16¶5.
- No pharmacokinetic studies were performed and “…are generally not considered necessary to support the development and licensure of vaccine products for infectious diseases (WHO, 2005, WHO, 2014).” P17¶1.
- “The biodistribution of the antigen encoded by the RNA component is expected to be dependent on the LNP distribution and the results presented should be representative for the vaccine RNA platform, as the LNP-formulated luciferase encoded modRNA had the same lipid compositions.” P17¶3.
- “Total recovery (%of injected dose) of LNP, for combined male and female animals, outside of the injection site was greatest in the liver (up to 18%) and was much less in the spleen (<1.0%), adrenal glands (<0.11%) and ovaries (<0.095%).
- “The protein encoded by the RNA in BNT162b2 is expected to be proteolytically degraded like other endogenous proteins. RNA is degraded by cellular RNases and subjected to nucleic acid metabolism. Nucleotide metabolism occurs continuously within the cell, with the nucleoside being degraded to waste products and excreted or recycled for nucleotide synthesis. Therefore, no RNA or protein metabolism or excretion studies will be conducted.” P20¶3.
“No PK drug interaction studies have been conducted with BNT162b2.” P.20¶4.
- Toxicology: Wistar Rat
- IM injection of BNT162b2 weekly for three weeks:
-
- Edema and erythema at the injection site.
- Transient elevation in body temperature.
- Elevations in WBC and acute phase reactants.
- Lower A:G ratios.
- Reduction in body weight.
- Reduction in Retic, PLT, and RBC mass parameters.
- Increased size of draining lymph nodes.
- Increased size and weight of the spleen.
- Increase cellularity in the draining lymph nodes, bone marrow, and spleen.
- Hepatocyte vacuolation in the liver.
- “All changes in clinical pathology parameters and acute phase proteins were reversed at the end of the recovery phase with the exception of higher RDW, higher globulins and lower A:G ratios in animals administered BNT162b2 (V9).”
- Single dose toxicity study was not done.
- Repeat dose toxicity: V8 data and not V9 are presented here: P23¶5 except for mention of lack of GGT elevation in V9 repeat dose study. This represents the only reporting on V9 on pages 24-26.
-
- Three weekly injections…” without evidence of systemic toxicity.” P23¶6.
- “…produced nonadverse macroscopic changes at the injection sites, spleen, and the draining lymph nodes; increased hematopoiesis in the bone marrow and spleen; periportal hepatocyte vacuolation; and clinical pathology changes consistent with those typically associated with ongoing recovery at the end of the 3-week recovery phase, and were consistent with those associated with the IM administration of LNP encapsulated mRNA vaccines.” P24¶1.
- Body weights were lower 24 hours after each V8 injection and normalized over study period.
- V8 group had higher temperatures at 4 and 24 hours after injection that did not exceed 40 degrees C.
- Incidence and severity of local edema and erythema were higher with each successive dose rising to the level of moderate to severe (rare). Resolved over study period.
- WBC cell counts were elevated: PMNs 7.8 x controls, eosinophils 5.1 x controls, basophil 1.47 x controls, Leucocytes 7.7 x controls. Highest on day 17, 48 hours after last injection. RBC retic counts reduced to 0.28 x controls and reduction in hgb. 0.87 x controls.
- Elevated GGT up to 4.6x controls on days 4 and 4.2x controls on day 17 for V8. “Additionally, higher GGT was not observed in the second repeat-dose toxicity study, conducted with the clinical candidate submitted for licensure.” P26 P23¶5.1.
- Albumin was down 0.87 x controls on day 4 and 0.76 x controls on day 17.
- Alpha-1-acid glycoprotein 21 x controls on day 17 and alpha-2-macroglobulin 217x controls on day 17 in both males and females.
- Fibrinogen was 3.1x higher than in controls.
- Spleen weights increased up to 1.62 x controls with increased lymphoid and/or hematopoietic cells.
- Microscopic findings were evident in injection sites, draining lymph nodes, bone marrow, spleen and liver.
-
-
- Injection site: infiltration of macrophages, granulocytes and lymphocytes in muscle, dermis and subcutis, moderate edema, myofibril degeneration, occasional muscle necrosis and mild fibrosis.
- Lymph nodes: increased cellularity with plasmacytosis variably present.
- Bone marrow: Increased (minimal to mild) cellularity.
- Spleen: increased hematopoiesis.
- Liver: Vacuolation (minimal to mild) thought to be caused by uptake of LNPs.
- Partial recovery at the injection site noted and to some extent in lymph nodes; spleen, bone marrow, and liver recovered fully at the end of the three-week recovery phase.
- CONCLUSION: 100 mg. x 3 “…was tolerated…without evidence of systemic toxicity.” P26¶6.
-
-
- 17-Day IM Toxicity study of BNT162b2 30 mg. x 3 (Days 1, 8, 15) in Wistar Han Rats with 3-week recovery. V9 data.
-
-
- Robust antigen-specific immune response.
-
-
-
- Nonadverse macroscopic changes at injection sites, spleen, lymph nodes, increased hematopoiesis in marrow and spleen, liver vacuolation with ongoing recovery at end of study period.
-
-
-
- Reduced food consumption 0.83x controls noted on days 4 and 11.
-
-
-
- Higher body temperature up to 0.54-degree C Day 1, up to 0.98-degree C Day 8 and up to 1.03 degrees C on Day 15.
-
-
-
- Injection site edema slight on Day 1, moderate on Day 8 and moderate on Day 15.
-
-
-
- Injection site erythema very slight on days 1, 8 and 15.
-
-
-
- Hematology:
-
-
-
-
- PMNs increased up to 6.60x controls,
- Monocytes up to 3.30x controls,
- Leucocytes up to 13.2x controls,
- Slightly higher eosinophils and basophils.
-
-
-
-
-
- Lower retics on day 4 down to 0.27x controls, up to 1.31x controls on day 17 in Females only.
- RBC mass down to 0.90x controls on Days 4 and 17.
- “…coagulation changes noted in the dosing phases were fully reversed after a 3-week recovery phase with the exception of higher red cell distribution width (up to 1.21x controls) …” P27 ¶5.
- A:G ratios down to 0.87x controls on Days 4 and 17.
- Fibrinogen levels up to 2.49x controls on Day 17.
-
-
-
-
- Spleen weights up to 1.42x controls in males and 12.59x controls in females return to WNL at end of study period.
-
-
-
- Lymph nodes: enlarged 2/20, pale/dark 5/20, firm 6/20 normalized at end of study period.
-
-
-
- Injection site: mixed cell inflammation mild to moderate and mild to moderate edema resolved at end of 3-week study period.
-
-
-
- Lymph nodes: up to moderate increase in cellularity of plasma cells and germinal centers. Partial recovery.
-
-
-
- Spleen and bone marrow: minimal increase in cellularity of hematopoietic cells. Partial recovery.
-
-
-
- Liver: vacuolation without hepatocellular damage. Complete recovery.
-
- Genotoxicity, Carcinogenicity, Reproductive and Developmental Toxicity:
- Genotoxicity: “No genotoxicity studies are planned for BNT162b2 as the components of the vaccine construct are lipids and RNA are not expected to have genotoxic potential (WHO 2005).” P29 ¶3.
- “Carcinogenicity studies with BNT162b2 have not been conducted as the components of the vaccine are lipids and RNA and are not expected to have carcinogenic or tumorigenic potential.” P29 ¶4. (WHO 2005)
- 30mg Wistar Han rats injected 21 and 14 days prior to mating and studied up to PND 21.
-
- “There were no BNT162b2-related effects on any ovarian, uterine, or litter parameters, including embryo-fetal survival, growth, or external, visceral, or skeletal malformations, anomalies, or variations.” P29¶8.
- No effects on postnatal offspring.
Not Studied:
- Secondary pharmacodynamics. P14¶1
- Safety pharmacology: “No safety pharmacology studies were conducted with BNT162b2 as they are not considered necessary for the development of vaccines according to the WHO guideline (WHO, 2005).” P.14¶2
- Pharmacodynamic Drug Interactions: “Nonclinical studies evaluating pharmacodynamic drug interactions with BNT162b2 were not conducted as they are not generally considered necessary to support development and licensure of vaccine products for infectious diseases (WHO, 2005).” P14¶3.
- No pharmacokinetic studies were performed with BNT162b2 and “…are generally not considered necessary to support the development and licensure of vaccine products for infectious diseases (WHO, 2005, WHO, 2014).” P17¶1.
- “The protein encoded by the RNA in BNT162b2 is expected to be proteolytically degraded like other endogenous proteins. RNA is degraded by cellular RNases and subjected to nucleic acid metabolism. Nucleotide metabolism occurs continuously within the cell, with the nucleoside being degraded to waste products and excreted or recycled for nucleotide synthesis. Therefore, no RNA or protein metabolism or excretion studies will be conducted.” P20¶3
- Genotoxicity: “No genotoxicity studies are planned for BNT162b2 as the components of the vaccine construct are lipids and RNA are not expected to have genotoxic potential (WHO 2005).” P29 ¶3.
- “Carcinogenicity studies with BNT162b2 have not been conducted as the components of the vaccine are lipids and RNA and are not expected to have carcinogenic or tumorigenic potential.” P29 ¶4. (WHO 2005)
- Phototoxicity. P30.
- Mechanistic studies. P30.
- Target organ toxicity: “Based on data from the GLP repeat-dose toxicity studies, administration of BNT162b2 was well tolerated without any evidence of systemic toxicity.” P31 ¶5.
- Dependence. P31.
- Metabolites. P31.
- Impurities. P31.
- Microanatomy studies of blood vessels, heart, lungs, and brain are not documented.
- Biodistribution of mRNA was not specifically studied.
- Biodistribution and toxicity studies of mRNA S protein were not referenced.
Preclinical Research Units:
| Study/Sponsor | |
| 1 | R-20-0085 |
| 2 | R-20-0112 |
| 3 | R-20-0211 |
| 4 | VR-VTR-10741 |
| 5 | VR-VTR-10671 |
| 6 | PF-07302048_06Jul20_072424 |
| 7 | R-20-0072: |
| 8 | 185350 |
| 9 | 01049-20008 |
| 10 | 01049-20009 |
| 11 | 01049-20010 |
| 12 | 01049-20020 |
| 13 | 01049-20021 |
| 14 | 01049-20022 |
| 15 | PF-07302048_05Aug20_043725 |
| 16 | 38166 |
| 17 | 20GR142 |
| 18 | 20256434 |
| 19 | 38166 |
| 20 | 20GR142 |
| 21 | 20256434 |




Why are they injecting people with ALC-0315?
https://i.postimg.cc/wvQ6J1cf/Stuff-In-The-Shots.png
In all caps lock “THIS PRODUCT IS FOR RESEARCH ONLY – NOT FOR HUMAN OR VETERINARY DIAGNOSTIC OR THERAPEUTIC USE”