Investigations
Report 98: FDA Selected Its ‘Vaccines Advisory Committee’ – Not Its Gene Therapy Advisory Committee – to Recommend the COVID Injections for Emergency Use, to Hide the Fact that the Products Are Not Vaccines But Gene Therapies
COVID-19 ‘vaccine’ drugs are modified mRNA gene therapy products. So, why did the FDA assign the review and recommendation responsibilities to the Vaccines and Related Biological Products Committee (VRBPAC) instead of the Cellular Tissue and Gene Therapy Advisory Committee (CTGTAC)? CTGTAC was the logical FDA advisory committee for such a …
Report 97: Pfizer Obscured Myocarditis Safety Signal Specific to Young Men. FDA Took Five Months to Notice Pfizer’s Obfuscation.
Career scientists are struggling with attention to detail in fulfilling their vaccine safety duties. Their conclusions, marked by errors and oversights, have led to lives lost and debilitating disorders, as proven by the government scientists’ own review documents. In spring 2021, the US Food and Drug Administration (FDA) granted emergency …
Report 96: mRNA COVID-19 Shots – ‘Vaccines’ or Gene Therapy Products? – Part 2
When considering a drug for emergency use authorization (EUA), the Food and Drug Administration (FDA) selects an internal advisory committee to review information and data submitted to the FDA by the pharmaceutical company about its new drug. These influential FDA advisory committees, based on that information and data, make recommendations …
Report 95: mRNA COVID-19 Shots – ‘Vaccines’ or Gene Therapy Products? – Part 1
To this day — more than three years after Pfizer and Moderna’s mRNA COVID-19 drugs received Emergency Use Authorization (EUA) — television, radio, and the Internet still inundate the public with ads, messages, and stories about COVID vaccines. But, are the mRNA COVID-19 shots genuinely vaccines? These products never aligned …
Report 94: Pfizer Secretly Studied a Heart Damage Marker, Troponin I, in Five- to 15-Year-Olds, Following mRNA COVID Vaccination in 2021.
We warned that there was proof that the Pfizer BNT162b2 mRNA COVID vaccine caused heart damage in teens and young adults, as early as May of 2021. As more information surfaces from the court-mandated release of Pfizer clinical trial documents by the FDA, and via FOIA’d emails, the CDC’s cover-up …
Report 93: Pfizer’s ‘Post-Marketing Surveillance Report’ Reveals That Pfizer Manipulated Data and Wrongly Tabulated Adverse Events, Which Concealed Them.
We evaluated Pfizer’s 38-page “5.3.6 Cumulative Analysis of Post-Authorization Adverse Events Reports of PF-07302048 (BNT162b2) received through 28-Feb-2021,” commonly known as “5.3.6,” starting in September of 2022. Patterns emerged across the System Organ Classes (SOCs). These patterns included Pfizer’s apparent concealment of various cases of adverse events, as well as …
Report 92: 100s of Possible Vaccine-Associated Enhanced Disease (VAED) Cases in First 3 Months of Pfizer’s mRNA COVID Vaccine Rollout, Yet Public Health Spokespeople Minimized Their Severity by Calling Them “Breakthrough Cases.”
By late February 2021, a group of experts called Brighton Collaboration released a paper, which was published in Vaccine, clearly defining Vaccine-Associated Enhanced Disease (VAED), which is a more severe clinical presentation of a disease in a person vaccinated against that disease than would normally be seen in an unvaccinated person. Yet, …
Report 91: FDA Based Moderna’s mRNA COVID Vaccine Approval on Test of a Completely Different Non-COVID Vaccine. Only Males Included in Test.
Moderna researchers did not test their COVID-19 mRNA drug, called SPIKEVAX, to find out where it would go in our bodies (biodistribution). Instead, their biodistribution study was for a completely different vaccine. Despite this substitution of one drug study for another, the U.S. Food and Drug Administration (FDA) approved SPIKEVAX …
Report 90: Pfizer’s ‘Post-Marketing Surveillance’ Shows mRNA-Vaccinated Suffered 1000s of COVID Cases in 1st 90 Days of Vaccine Rollout. Most Infections in the Vaccinated Categorized as ‘Serious Adverse Events.’
Though spokespeople assured us that the COVID-19 injection stops — well — COVID, Pfizer and the Food and Drug Administration (FDA) both knew that during the first three months of Pfizer’s mRNA COVID-19 vaccine rollout, thousands of COVID cases were reported to Pfizer — among vaccinated people. The ‘COVID-19 Adverse …
Report 89: BOMBSHELL – War Room/DailyClout Researchers Find Pfizer Delayed Recording Vaccinated Deaths at Critical Juncture of EUA Process. Improper Delays in Reporting Deaths in the Vaccinated Led FDA to Misstate Vaccine’s Effectiveness, Influenced EUA Grant Decision.
On September 5, 2023, DailyClout reported that a War Room/DailyClout Research Team – Corinne Michels, PhD; Daniel Perrier; Jeyanthi Kunadhasan, MD; Ed Clark, MSE; Joseph Gehrett, MD; Barbara Gehrett, MD; Kim Kwiatek, MD; Sarah Adams; Robert Chandler, MD; Leah Stagno; Tony Damian; Erika Delph; and Chris Flowers, MD – broke …
Report 88: 2.5 Months After COVID Vaccine Rollout, Pfizer Changed Criteria for ‘Vaccination Failure,’ Causing 99% of Reported Cases to Not Meet That Definition. 3.9% of Reported ‘Lack of Efficacy’ Cases Ended in Death in First 90 Days of Public Vaccine Availability.
The War Room/DailyClout Pfizer Documents Analysis Project Post-Marketing Group (Team 1) – Barbara Gehrett, MD; Joseph Gehrett, MD; Chris Flowers, MD; and Loree Britt – penned a telling analysis of the “Vaccine Effectiveness” Safety Concern section found in Pfizer document 5.3.6 Cumulative Analysis of Post-Authorization Adverse Event Reports of PF-07302048 …
Report 87: In Early 2021, Pfizer Documented Significant Harms and Deaths Following Vaccination with Its mRNA COVID Vaccine. The FDA Did Not Inform the Public.
INTRODUCTION “5.3.6 Cumulative Analysis of Post-Authorization Adverse Event Reports of PF-07302048 (BNT162b2) Received Through 28-Feb-2021,” Pfizer’s post-marketing report, a required U.S. Food and Drug Administration (FDA) compliance document, is one of the ways in which the FDA assessed patients’ risks associated with Pfizer’s COVID-19 vaccine, BNT162b2. The data within the …
Report 86: Pfizer’s Clinical Trial ‘Process 2’ COVID Vaccine Recipients Suffered 2.4X the Adverse Events of Placebo Recipients; ‘Process 2’ Vials Were Contaminated with DNA Plasmids.
Process 2 was hidden all along in Pfizer’s COVID ‘vaccine’ clinical trial, and the War Room/DailyClout investigators’ findings about it are mind-blowing. The Food and Drug Administration (FDA) knew that the Process 2 subjects had very high levels of adverse events, but there is no evidence that the agency acted …
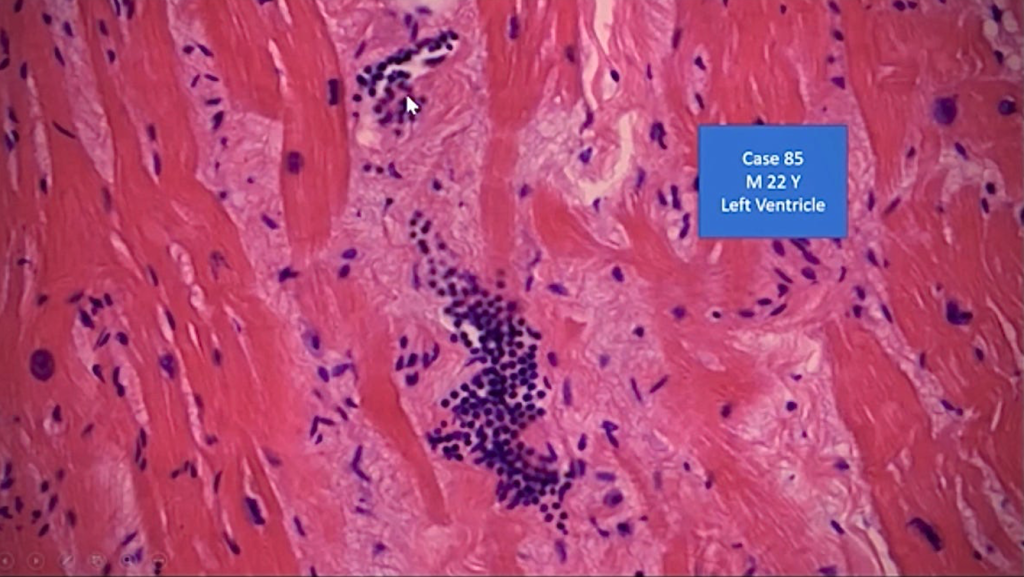
Report 85: “The Underlying Pathology of Spike Protein Biodistribution in People That Died Post COVID-19 Vaccination” – Dr. Arne Burkhardt
The Scientific and Medical Advisory Committee hosted a meeting with special guest speaker Professor Arne Burkhardt. Dr. Arne Burkhardt Compiled and edited by Robert W. Chandler MD MBA* *A transcript was produced then edited with a diligent effort to leave the meaning unchanged. My additions are in italics. …
Report 84: War Room/DailyClout Research Team Breaks Huge Story: More Cardiovascular Deaths in Vaxxed Than Unvaxxed; Pfizer Did Not Report Adverse Event Signal; Death Reporting Delays Favored Pfizer/Vaccinated.
War Room/DailyClout Pfizer Documents Analysis team members Corinne Michels, PhD; Daniel Perrier; Jeyanthi Kunadhasan, MD; Ed Clark, MSE; Joseph Gehrett, MD; Barbara Gehrett, MD; Kim Kwiatek, MD; Sarah Adams; Robert Chandler, MD; Leah Stagno; Tony Damian; Erika Delph; and Chris Flowers, MD have published a bombshell study titled, “Forensic Analysis …
Report 83: 23% of Vaccinated Mothers’ Fetuses or Neonates Died. Suppressed Lactation and Breast Milk Discoloration Reported.
The War Room/DailyClout Pfizer Documents Analysis Project Post-Marketing Group (Team 1) – Barbara Gehrett, MD; Joseph Gehrett, MD; Chris Flowers, MD; and Loree Britt – wrote a shocking analysis of the “Use in Pregnancy and Lactation” section found in Pfizer document 5.3.6 Cumulative Analysis of Post-Authorization Adverse Event Reports of …
Report 82: Pfizer Clinical Trial Subject Dies Soon After Receiving One Dose of Moderna COVID Vaccine. Forty-Two Days Post-Mortem, Pfizer Staff Seem Unaware He Is Dead.
A protocol keeps a clinical trial accurate and rigorous. The 361-page Case Report Form (CRF) for Pfizer clinical trial subject 10841470 shows that Pfizer allowed significant deviations from its protocol, such as not discontinuing the subject from the trial when he first took a different brand of COVID vaccine and …
Report 81: Summary of 2.4 Nonclinical Overview – Pfizer mRNA COVID-19 Vaccine, BNT162b2
Reference document Pfizer confidential document “2.4 NONCLINICAL OVERVIEW,” Version 3 (36 pages) while reading this summary. [https://phmpt.org/wp-content/uploads/2022/03/125742_S1_M2_24_nonclinical-overview.pdf] Table of Contents pps. 2-3 Abbreviations pps. 4-5 Nonclinical Testing Strategy Overview pps 6-9 Pharmacology pps. 10-11 Immunogenicity pps. 11-13 Pharmacokinetics pps. 15-20 Toxicology pps. 21-29 Genotoxicity, Carcinogenicity, Reproductive and Developmental Toxicity pps. …
Report 80: Moderna mRNA COVID-19 Injection Damaged Mammals’ Reproduction: 22% Fewer Pregnancies; Skeletal Malformations, Pain, Nursing Problems in Pups. FDA Knew, Yet Granted EUA.
Based on “A GLP intramuscular combined developmental and perinatal/postnatal reproductive toxicity study of mRNA-1273 in rats. FDA-CBER-2-22-4207-0015,” Moderna’s COVID-19 mRNA drug damaged the reproductive systems of female rats their estrous cycles (the recurring period of sexual receptivity and fertility in many female mammals) were disturbed, they were less likely to …
Report 79: mRNA COVID Vaccine-Induced Myocarditis at One Year Post-Injection: Spike Protein, Inflammation Still Present in Heart Tissue.
In spite of widespread censorship, the truth is coming out that myocarditis arising after injection with mRNA COVID “vaccines” is not rare, temporary, or mild. https://openvaers.com/covid-data/myo-pericarditis Statement 1: Myocarditis after COVID-19 “vaccine” injection is rare. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html The Centers for Disease Control and Prevention’s (CDC) notice and its …