Report 100: Pathological Basis of CoVax Disease Cardiovascular Manifestations
Republished with Permission from Doctors for COVID Ethics
This document contains summaries of two autopsy cases that were examined by Pathologists Arne Burkhardt and Walter Lang at Reutlingen, Germany. These summaries were translated and edited from Dr. Burkhardt’s own files.
Two cases are presented that demonstrate catastrophic damage to the cardiovascular system from the COVID19 gene therapy drugs. Both had fatal outcomes, one by suicide.
I. Aortic Aneurysm with Cardiac Tamponade and Myocarditis (Burkhardt/Lang Case #10)
- History
6/25/2021 BNT162b2 Dose #1 Lot1D017A
6/08/2021 BNT162b2 Dose #2 Lot FF3318
This 61-year old man received BNT162b2 because vaccination was required for travel. Three weeks after his first dose of BioNT162b2 he “fell off chair” and was taken by ambulance to the hospital where he was diagnosed as having dehydration.
He subsequently had sudden onset of chest pain and collapsed 25 days after dose 2 of BioNT162b2. Attempts to resuscitate him by his partner present at the time failed, as did attempts at resuscitation by an emergency doctor. Echocardiography revealed pericardial tamponade from clotted blood.
- Past Medical History
- Myocarditis 30 years previously following
- Hypertension
- Smoker
- Body Mass Index 1 (overweight)
C. Histopathology
| Organ | Findings | Cell Types |
|
1. Aorta |
Vasculitis with media necrosis, dissection and rupture with pericardial tamponade, destruction of elastic lamellae, focal dense inflammatory infiltration, perivascular inflammation of vasa vasorum | Myofibroblasts Inflammatory cells Lymphocytes |
| 2. Heart | Endothelialitis, pericarditis, focal myocarditis, vasculitis, diffuse scarring | Lymphocytes |
| 3. Kidneys | Lymphocytic infiltration | Lymphocytes |
| 4. Liver | Spike protein in vessel walls, vasculitis | Lymphocytes |
| 5. Lung | Lymphocytic infiltration | Lymphocytes |
| 6. Spleen | Spike protein in vessel walls with perivascular onion skin formation, spike diffuse in parenchyma, vasculitis | Lymphocytes |
| 7. Testes and Epididymis | Lymphocytic infiltration | Lymphocytes |
D. Cause of Death:
Ruptured aorta with pericardial tamponade. BioNTech vasculitis, dissecting aneurysm aorta with myocardial fibrosis from prior post influenza myocarditis. Multiorgan involvement.
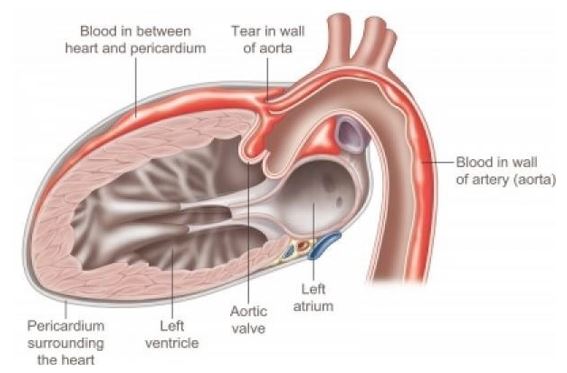
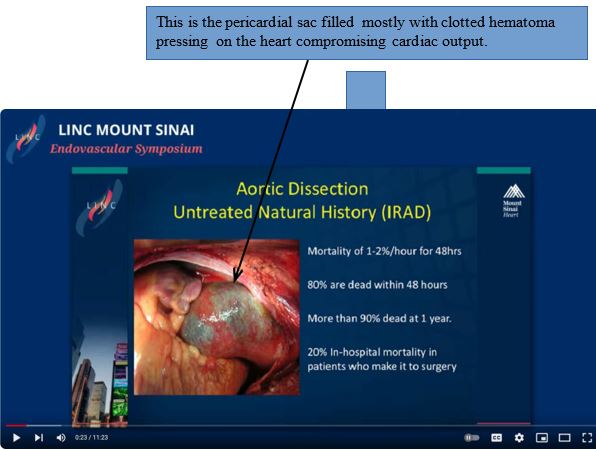
This drawing (from https://www.pinterest.com/pin/859906122587268332/) illustrates the medical condition that caused the death of this patient. A tear in the wall of the aorta causes blood to enter in between the layers of the aortic wall itself, as well as into the pericardium, the fibrous sac that surrounds the heart. The blood in the pericardium compresses the heart and thus disables its function.
E: Histopathological Documentation
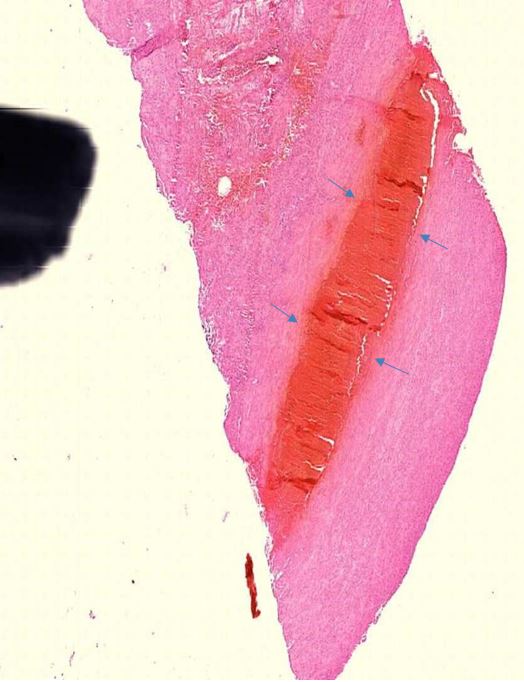
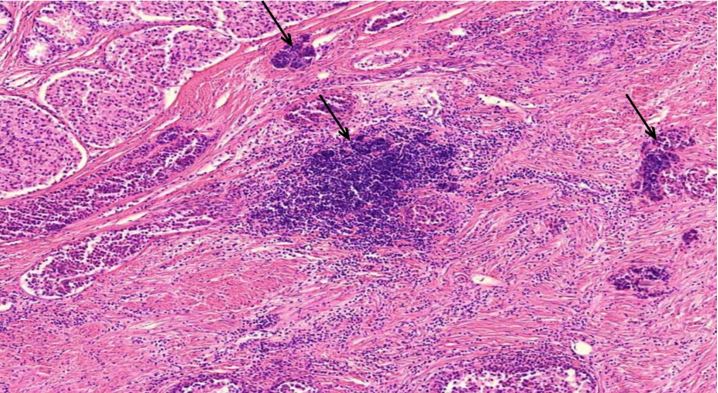
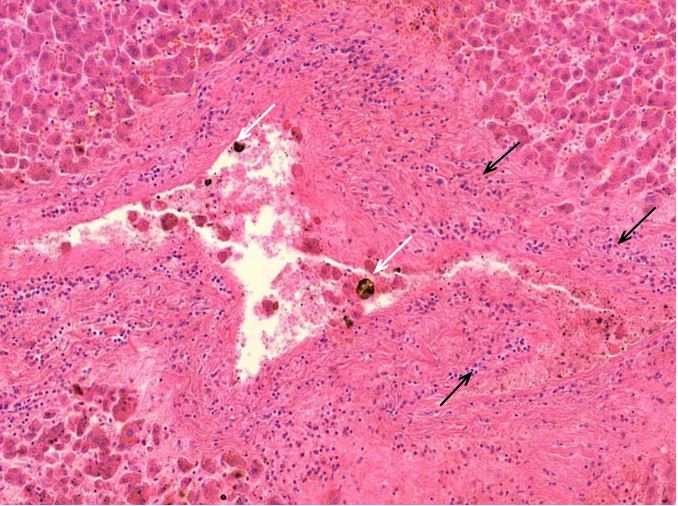
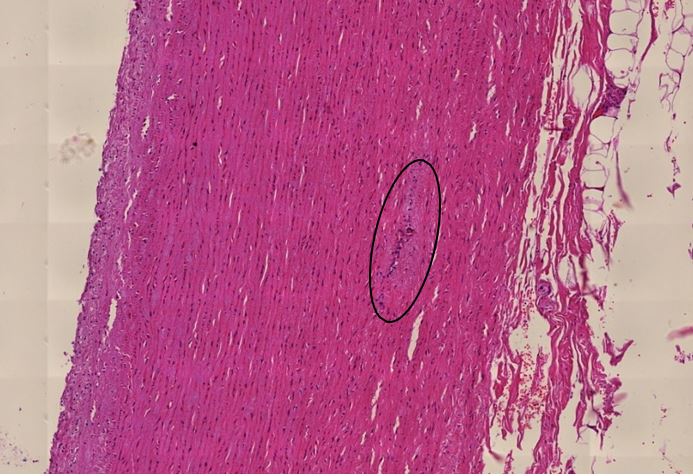
Figure 1.1 Large vessel, dissection.
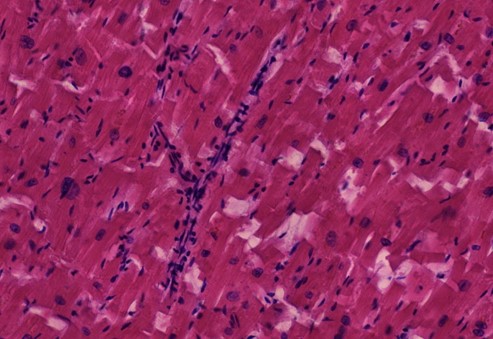
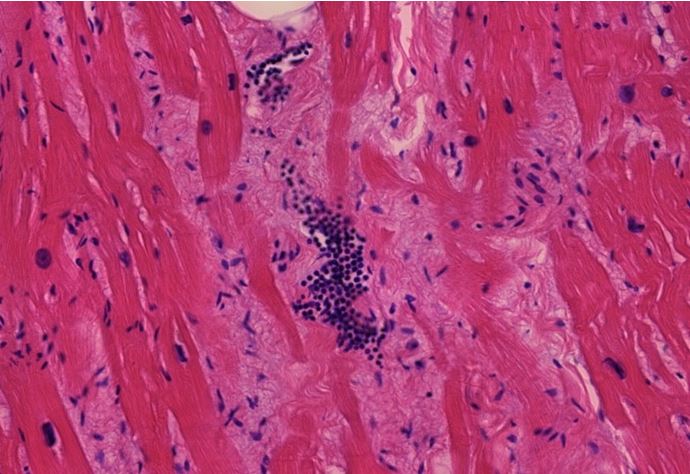
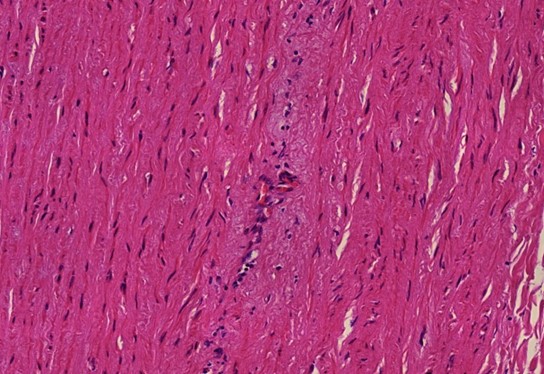
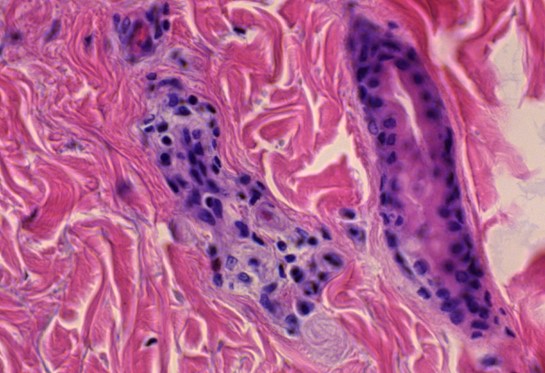
Figure 1.2 Aorta, vasculitis, media necrosis with lymphocytic infiltration. The smooth muscle tissue of the vessel wall shows discontinuities and the surrounding tissue areas show clusters of lymphocytes, which appear small, round and blue. Outright dissection occurs when such necrotic lesions coalesce and become a cleavage plane through which blood forced by pressure from the contracting heart muscle.
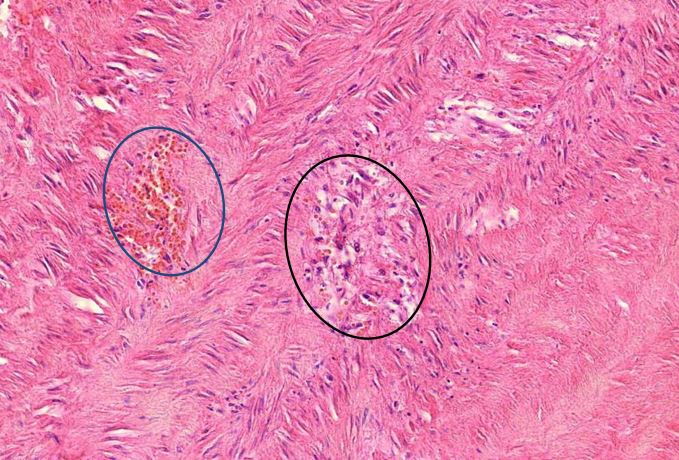
Figure 1.3 Large vessel, media necrosis. Blood has seeped into one lesion (blue outline), whereas the other shows disturbances of the tissue structure (black outline)
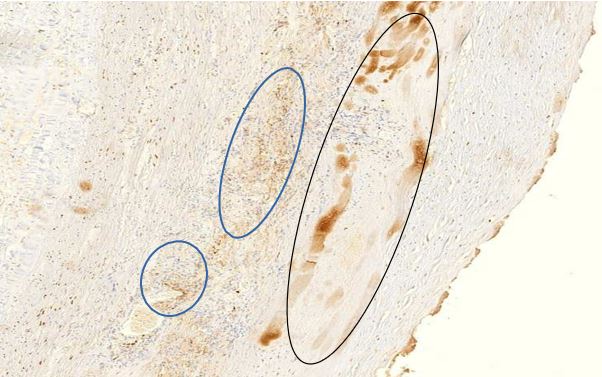
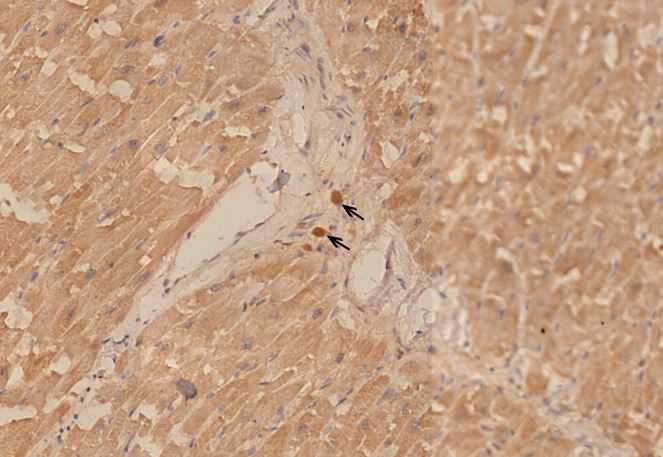
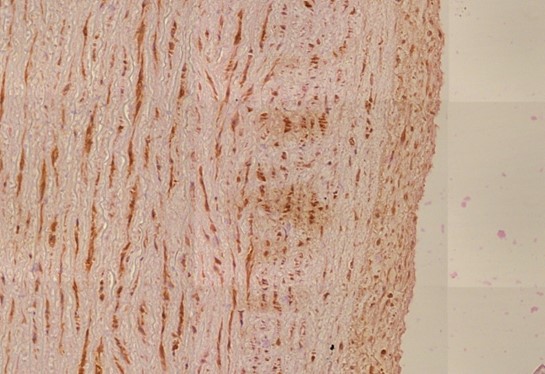
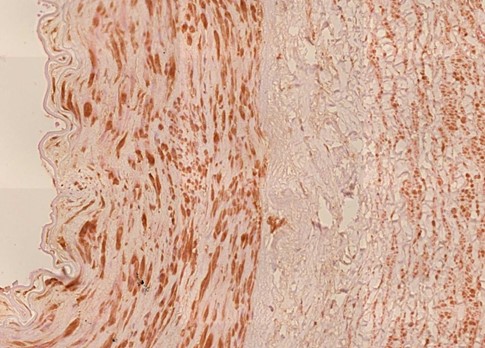
Figure 1.4 Aorta, tunica media vasculitis, spike protein immunohistochemistry. The vaccine-induced expression of spike protein in the tissues can be detected using specific antibodies which in this image is demonstrated by a brown pigment indicating antibody binding to Spike protein. For an explanation of the technique, see https://www.youtube.com/watch?v=zO70nrWcAyk.
Blue outlines: clusters of cells are stained brown, indicating the presence of spike protein. Black outline: the large “blobs” are artifacts.
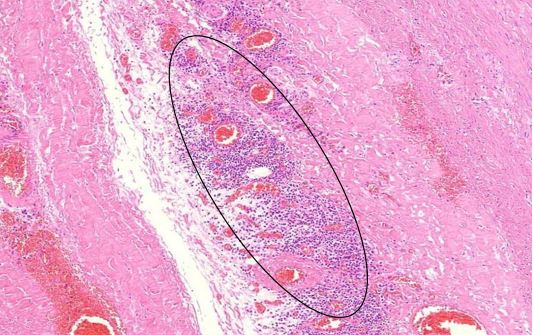
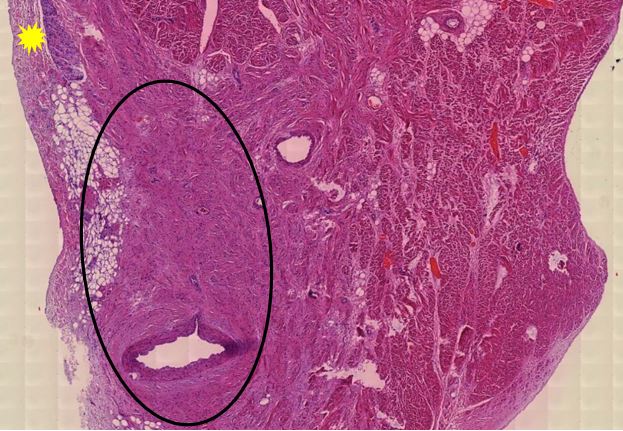
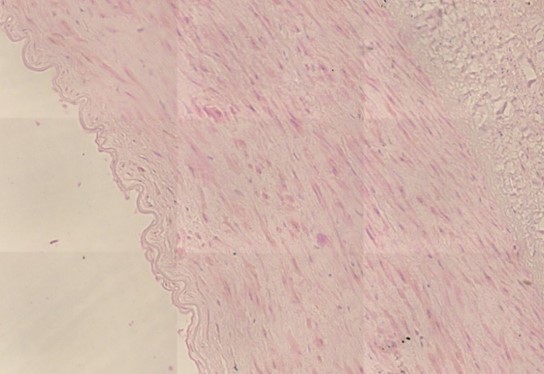
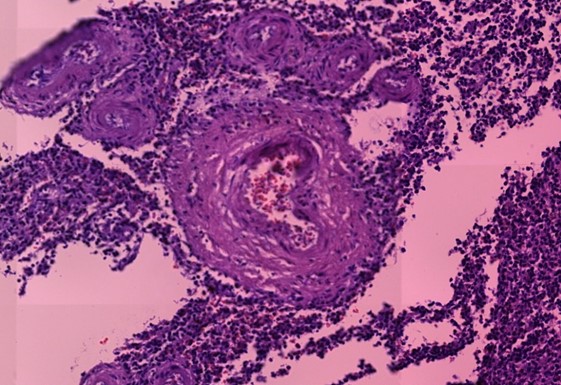
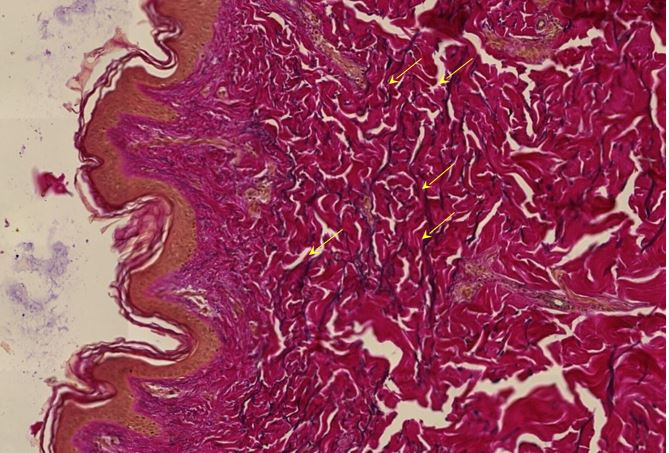
Figure 1.5 Aorta, adventitia, perivasculitis. The small blood-filled vessels are the vasa vasorum, which penetrate the aortic wall from the outside to supply it with blood. The tissue surrounding these vessels is heavily infiltrated with lymphocytes.
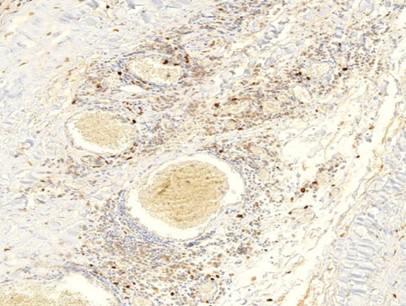
Figure 1.6 Aorta, perivasculitis, spike protein immunohistochemistry. The brown pigment is found primarily within inflammatory cells.
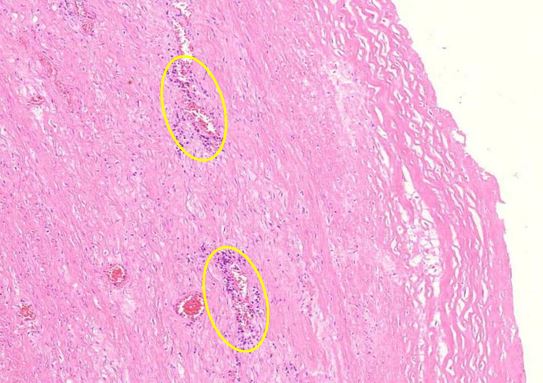
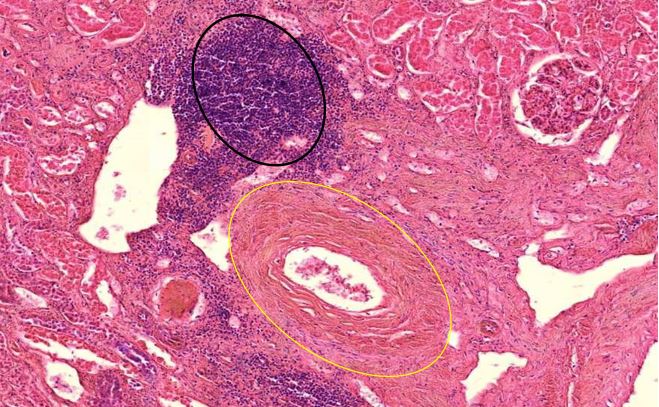
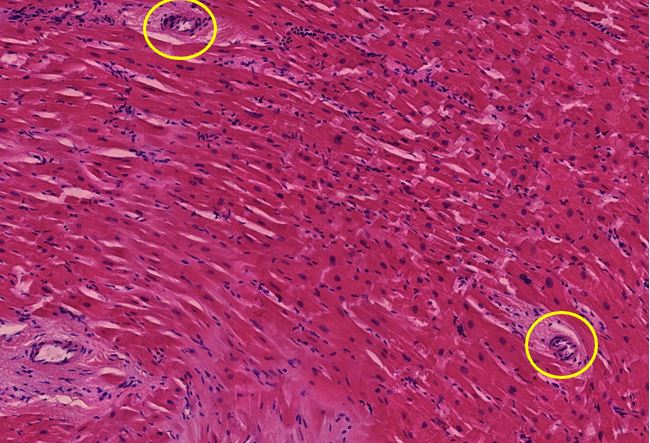
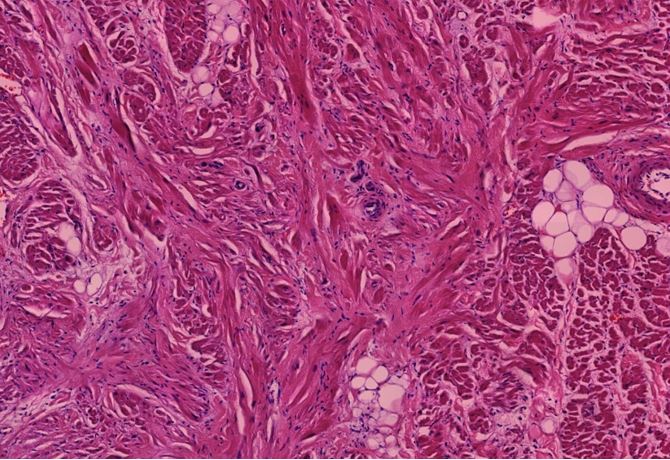
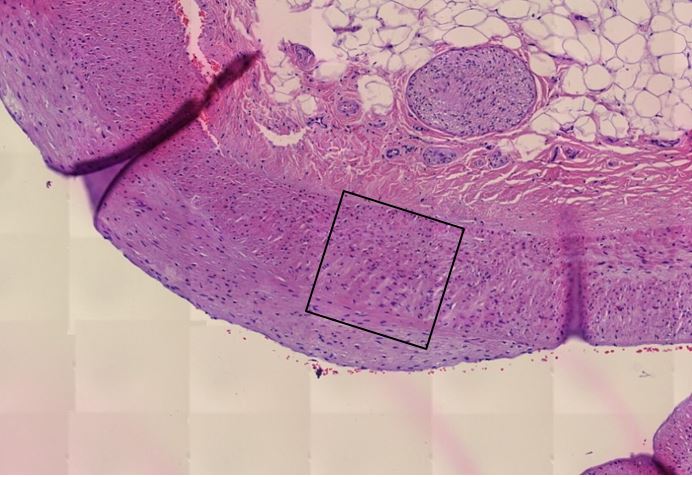
Figure 1.7 Kidney, interstitial lymphocytic infiltration (black oval), vasculitis with onion-skin aspect (yellow oval). The onion-skin phenomenon signals that the normally compact muscular layer of the vessel wall has been fragmented into several concentric sheets of tissue, representing chronic injury; older than 48 hours, cannot rule out injury related to the vaccination due to the medical history.
Figure 1.8 Testis, lymphocytic infiltration, vasculitis.
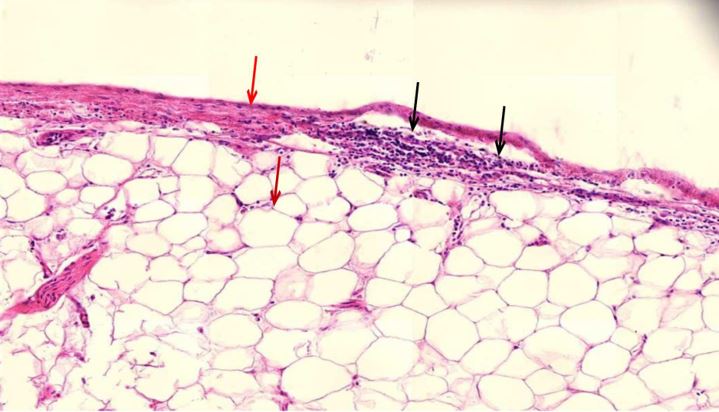
Figure 1.9 Heart, the epicardial lymphocytic infiltration. The epicardium (red arrow) is the layer of epithelial tissue that covers the heart. Beneath it is a layer of fat tissue (lower red arrow), the fat cells appear empty, the fat washed out during the tissue processing. Here, the epithelial layer invaded by lymphocytes (black arrows).
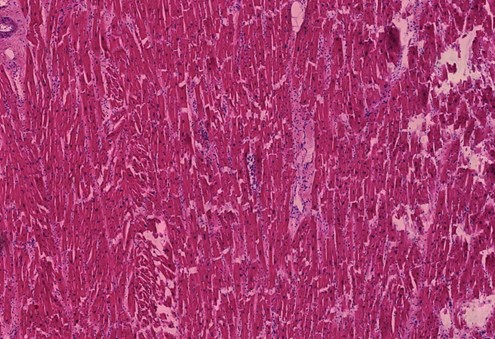
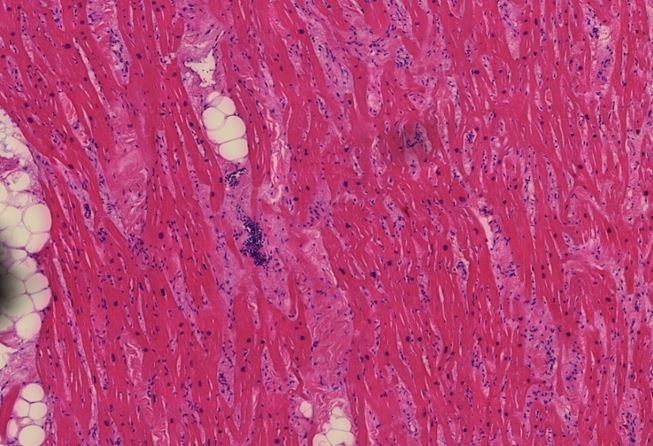
Figure 1.10 Heart muscle, diffuse scars, slight lymphocytic inflammation. The heart muscle cells appear bright red, whereas the scar tissue is pale red. Scattered blue round lymphocytes seen throughout.
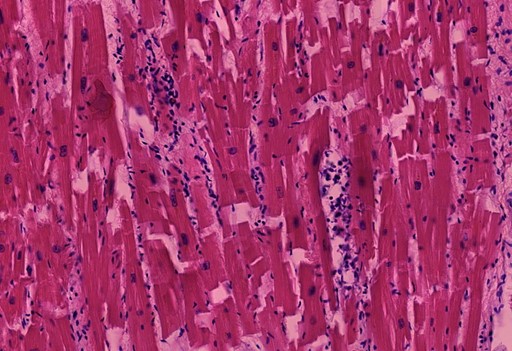
Figure 1.11 Heart, vasculitis of a small blood vessel. The vessel wall innermost layer cells sloughed off filling the lumen. The endothelial desquamation and luminal vessel lymphocytes present indicate the pathological injury (inflammation) during life rather than postmortem tissue decay. (arrows)
Figure 1.12 Liver, vasculitis. Regular (epithelial) liver cells seen in the two top corners and at the bottom left. The greater part of this image filled by the cross-section of a large blood vessel wall densely infiltrated with lymphocytes. (black arrows).
The reddish brown oval to round shapes (white arrows) are suggestive of free or oxidized hemoglobin deposits due to the erythrocyte damage and lysis that at the end can cause toxic chemical endothelial cell injury.
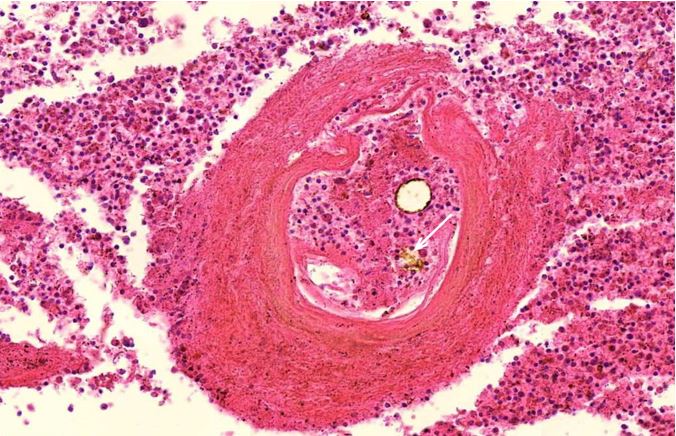
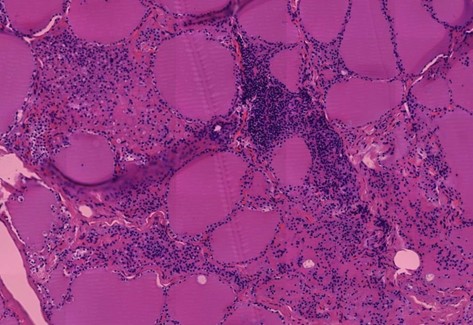
Figure 1.13 Spleen, vasculitis with onion-skin pattern of the vessel wall. Burkhardt and Lang found this onion-skin pattern most commonly in blood vessels of the spleen. The white arrow indicates free or oxidized hemoglobin in the lumen of the vessel..
F. Dr. Burkhardt and Dr. Lang’s Summary and Interpretation
Summary
In the 26 organ samples, in addition to age-appropriate changes, typical Corona vaccination-associated organ changes were found consisting of:
- Minor endothelialitis, minor pericarditis, focal myocarditis in the heart
- Lymphocytic infiltrates outside lymphoid organs, especially in testicles and epididymis, lungs, kidneys, and spleen.
- In the spleen, the typical onionskin-like loosening of the vessel walls with clear expression of spike protein are particularly impressive. In addition, spike expression observed diffusely within the entire splenic parenchyma.
- Within the liver, spike expression is found within vascular wall cells of blood vessels, but not within the epithelial hepatocytes.
The overall histological findings in conjunction with the very informative immunohistochemical analysis prove that the patient has pronounced Covid impregnated organ changes, in particular an excessive immune reaction.
The cause of death, as already established in the initial autopsy, is clearly a dissection of the ascending aorta with wall rupture and bleeding.
However, in addition to the undoubtedly existing atherosclerotic changes, the present sectional specimens show a profound texture disorder of the aorta with media necrosis, dissection, destruction of elastic lamellae, focal dense inflammatory infiltrations, as well as considerable perivascular inflammation of the vasa vasorum.
Deep-seated media necrosis does not fit the picture of regular atherosclerosis but speaks for an inflammatory toxic genesis. In keeping with this, the spike protein detected in the aortic wall, in myofibroblasts, in inflammatory cells, and perivascularly around the vasa vasorum.
The histological picture does not correspond to the usual atherosclerotic dissection and rupture. There is also no picture of idiopathic microcystic media necrosis, nor is there any indication of a chronic inflammatory process.
Interpretation
The pattern of damage to the aorta corresponds to changes after intoxication (e.g., lathyrism). In fact, the toxic spike protein, which is Covid vaccine-induced, selectively detected in damaged aortic sections with dissection and rupture.
“This means that the death caused by bleeding after aortic rupture must be clearly classified as a consequence of vaccination.” Arne Burkhardt MD, Walter Lang MD
G. Clinical Context
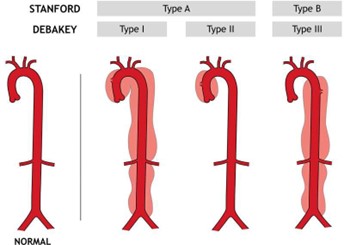
This 61 year-old man had rupture of his aorta at a point right next to the aortic valve, which separates the heart’s left ventricle and the aorta. Because of this, bleeding occurred not only into the aortic wall but also into the pericardial sac. The resulting compression of the heart was most likely the immediate cause of death. The ascending aorta aneurysms are classified by cardiothoracic surgeons as Type A.
https://healthjade.com/aortic-dissection/
Even if they are not immediately life threatened as in this case, type A aneurysms present a challenge for operative repair due to proximity to the aortic valve and involvement of major
arteries that take off from the aorta as the aorta transitions from ascending to descending. Pathology in this region of the aorta carries a high mortality rate of 80% within 48 hours and 90% at one year according to Dr. Allen Stewart, cardiothoracic surgeon, formerly of Mount Sinai Hospital.
https://www.youtube.com/watch?v=dZYg5xoawyQ
In this video, Dr. Stewart discusses the surgical repair along with the prognosis that is not good.
https://www.youtube.com/watch?v=UaGJYJfe_Nk
In this video, Dr. Yan has removed the clot from inside the pericardial sac and proceeds to repair the aneurysm.
In addition to this man’s catastrophic aneurysm, he had histologic evidence of post BNT162b2 inflammation with lymphocytic infiltration of his heart, kidneys, liver, lung, spleen, and testes.
II. Acute and Chronic Myocarditis, Early Aortic Coronary Artery Aneurysm (Burkhardt/Lang Case #85)
A. History
08/26/2021 Janssen Lot 21C18-03 Dose #1 death 364 days later 12/30/2021 BNT162b2 Lot PCA0003 Dose #2 death 238 days later
This 22 year-old male was a competitive swimmer (40-50 km) who noticed a sharp drop in his performance following the COVID injections. He had his last dose of LNP/modRNA on 12/30/2021. He was unable to swim competitively from April 2021 onward. He committed suicide on August 25, 2021 not wanting to live with myocarditis.
B. Past Medical History
Unknown
C. Histopathology
| Organ | Findings | Cell Types |
|
1. Aorta |
Deep seated media necrosis with isolated lymphocytic infiltrates and bleeding between the outermost media and adventitia, and curling of the elastin fibers. Isolated endothelial swelling on the vasa vasorum with perivascular infiltrate. Spike + Nucleocapsid – in vascular endothelia and myofibroblasts of the media.
Nucleocapsid negative. Berlin blue negative for hemosiderin pigment, no CD3 or CD20. ECG: Disturbance of the vascular wall architecture. Berlin blue: Neg. for hemosiderin pigment and CD3, CD20 |
Lymphocytes |
|
2. Heart |
Coronary artery: No ASCVD plaques, slight lymphocytic inflammation of the vaso vasorum with endothelial swelling. Spike S1 IHC Significant positivity of endothelia and myofibroblasts in the inner wall and outer layers. In the media partial necrosis. Nucleocapsid
– Berlin blue: no hemosiderosis// Posterior septum: Endothelialitis in intramural capillaries Endocardial area with mild interstitial fibrosis. Marked myofibroblast proliferation.//Spike S1 IHC: Spike markings in the endothelia of the arterioles, focal marking of myocytes, perivascularly individual, strongly labeled inflammatory cells (monocytes). Texture disorder of an epicardial smaller coronary artery branch with marking of myofibroblasts. Nucleocapsid IHC -//Right ventricle: extensive scarring around a small coronary artery branch. |
Lymphocytes, plasma cells, and myofibroblasts |
| myocardial damage and not ischemic. In periphery of scar thinning lymphocyte infiltrate and myofibroblast activation. CD 31.// Left ventricle: florid myocarditis with partially denser interstitial infiltrates of lymphocytes, plasma cells, and myofibroblast proliferation, isolate muscle fiber loss, necrotic muscle fibers and fibroblast proliferation//Spike S1IHC marking of endothelia and focal marking of myocytes.
Nucleocapsid -. |
||
| 3. Lung | Interstitial focus with foreign giant cells and anthracitic
pigment deposits |
Giant cells |
| 4. Lymph node | Anthracitic pigment increased cellular and humoral
immune response. |
Mixed |
| 5. Pancreas | Focal vacuoles with sparse brown-black rod-shaped
particles |
|
|
6. Skin |
Subepithelial swelling and perivascular infiltration, Swollen endothelium, partly detached from the vessel wall with strong positive reaction to endothelial cell marker CD31. Lympho-plasma cellular inflammation. ECG: rarefaction of basement membrane-associated
network. Coarsening and fragmentation of elastic fibers. |
Lymphoplasma cells |
| 7. Spleen | Activated follicles, onionskin pattern hinted at. | Lymphoid tissue |
| 8. Thyroid | Focal lymphocytic thyroiditis | Lymphocytes |
D. Histopathological Documentation
Figure 2.1: Heart, posterior septum, lymphocytic infiltration
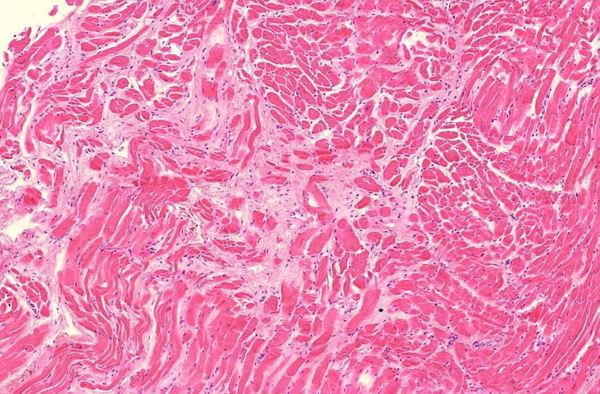
Figure 2.2: Heart, left ventricle, myocarditis with scars, interstitial edema and inflammatory infiltrates
Figure 2.3: Heart, left ventricle, detail of preceding image
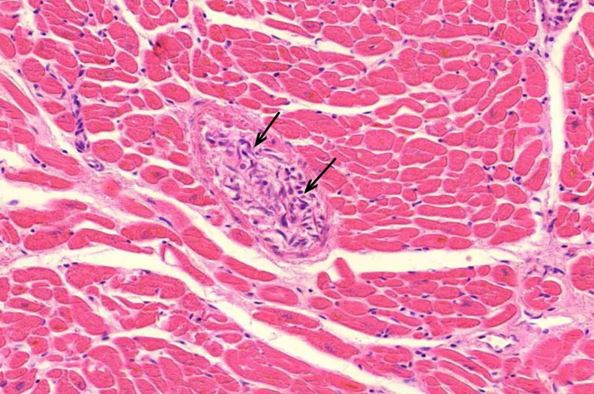
Figure 2.4: Heart, left ventricle, myocarditis with interstitial edema and inflammatory infiltration, vasculitis of small vessels (yellow circles)
Figure 2.5: Heart, right ventricle, myocarditis with infiltration and advanced fibrosis
Figure 2.6: Heart, right ventricle, detail of preceding image
Figure 2.7: Heart, right ventricle, extensive scar surrounding a coronary artery branch. The yellow star sits to the left of a site of activated lymphocyte-mediated inflammation.
Figure 2.8: Heart, right ventricle, detail from peripheral zone of scar shown in previous image
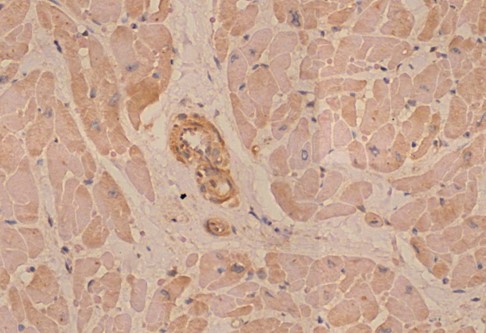
Figure 2.9: Heart, posterior septum, spike protein immunohistochemistry (IHC). Black arrows point to cells that are probably macrophages.
Figure 2.10: Heart, posterior septum, spike protein IHC. Both muscle fibers and the wall of a small blood vessel are stained brown, indicating spike protein expression.
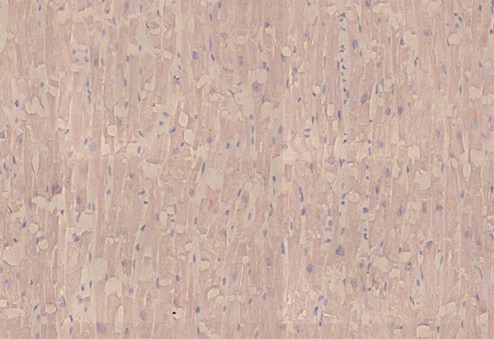
Figure 2.11: Heart, posterior septum, nucleocapsid IHC. The absence of brown stain indicates that the nucleocapsid protein is not expressed, which it should be in an infection with SARS-CoV-2. This confirms that the expression of spike protein in this case caused by vaccination rather than virus infection.
Figure 2.12: Aorta, deep media necrosis with lymphocytic infiltration (black oval).
Figure 2.13: Aorta, detail of preceding image
Figure 2.14: Aorta, immunohistochemical (IHC) stain indicates the presence of spike protein.
Figure 2.15: Aorta, nucleocapsid IHC negative, therefore not related to SARS-CoV-2 but rather C19 vaccine.
Figure 2.16: Coronary artery, vasculitis of vasa vasorum media. Black box shows expansion of a zone of cellular and intracellular edema.
Figure 2.17: Coronary artery, spike protein is demonstrated by IHC staining.
Figure 2.18: Spleen, vasculitis with onion-skin injury that is a minimum of 14 days old. Here one can see the artery is edematous in all layers, similar that was described in Figure 2.16.
Figure 2.19: Thyroid gland, lymphocytic inflammation. The large, homogeneously stained follicles are the place at which the initial stages of thyroid hormone synthesis occur. The infiltrate of lymphocytes is located in the interstitial tissue between the follicles. These findings are consistent with autoimmune thyroiditis.
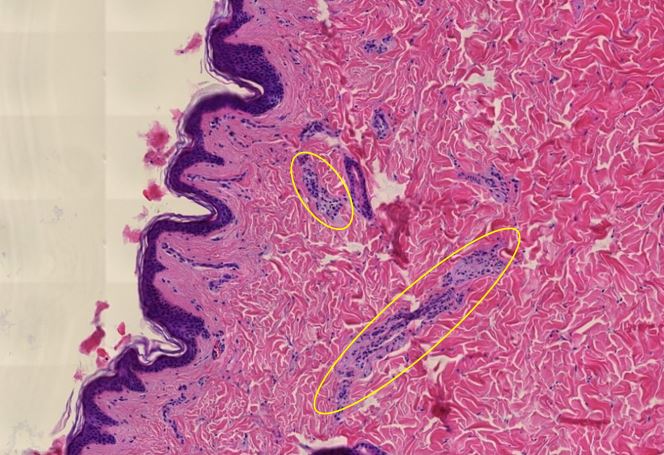
Figure 2.20: Skin, vasculitis of dermal vessels (yellow ovals).
Figure 2.21: Skin, detail of preceding image
Figure 1.22: Skin, Elastica Van Gieson stain, fragmentation of elastic fibers, which stand out by their dark color
E. Dr. Burkhardt/Lang’s Summary and Interpretation
The main finding of the deceased was a severe, partly florid, partly subsided, and scarring myocarditis with an existing acute relapse at death. The focus of the scarring was in the right ventricle.
In this case, there is a multifocal event in the heart muscle. A heart attack was ruled out as the cause of the scarring:
- No coronary sclerosis
- Multiple disease foci
- Predominantly lymphocytic infiltrate
Marked texture disorders of the walls of large arteries and the aorta with median swelling and discrete inflammation of the vasa vasorum. The discrete peri-vasculitis in the skin and a (only slightly pronounced “onion skin phenomenon” of the splenic arteries) fit into the picture.
With a probability bordering on certainty, there was severe corona vaccination-related organ damage at the time of suicide. This confirmed by the positive spike detection in the damaged organ structures, especially the heart muscle, coronary arteries, and aorta, with negative findings for nucleocapsid immunohistochemistry.
G. Clinical Context
Drs. Burkhardt and Lang did not record reasons why this unfortunate young athlete was not offered a heart transplant, possibly because he was too early in the process. Perhaps the young man’s decision to take his life might have been a result of his other manifestations of Spike related damage to other organs such as his lungs, skin, spleen, and thyroid. He may not have known about the pathology in his aorta and coronary artery.
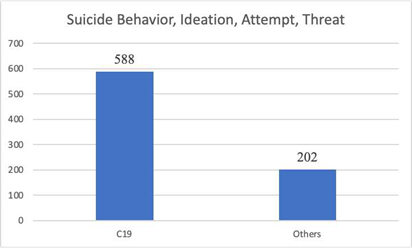
Sudden onset, complex illness has a devastating psychological impact. The Vaccine Adverse Event Reporting System contains 790 reports of suicidal behavior, ideation, attempt and threat for all known “vaccines” 74% of which were following COVID19 gene therapy products. In 15 of these cases death occurred, 12 following COVID19 GTPs and 3 after other products.
III. CoVax and Turbo CoVax Disease
These two cases document destructive multiple organ system lymphocytic infiltration following spike producing injectable products. Spike protein is demonstrated in the tissues with no evidence of SARS- CoV-2 nucleocapsid protein, indicating that the spike-mediated tissue damage was due to vaccination and not COVID infection. The two experienced pathologists – emeritus professors of pathology – listed the COVID19 spike producing products as the cause of profound vascular and myocardial disease that directly led to one death and indirectly to the second.
In addition to the catastrophic damage to their cardiovascular systems, both men had multiple organ systems showing histopathological and immunohistochemically evidence of damage from COVID19 vaccines. Even if the 61-year-old man had made it to the operating room to have his cardiac tamponade relieved and his aneurysm repaired his prognosis was poor. The younger man might have undergone successful heart replacement at some future date only to discover he also needed repair of an aortic aneurysm later as well as shown in case 1.
In their notes on Case 2, Drs. Burkhardt and Lang used the term “Covid impregnated” but we prefer the term ”CoVax” as it ties more directly to the vaccine rather than the disease from SARS-CoV-2 virus as Drs. Burkhardt and Lang have clearly documented vaccine damage in these two cases. Disease states that result from the spike producing injectable products can then be termed CoVax Disease and aggressive manifestations of CoVax Disease are termed Turbo CoVax Disease as in the already widely used Turbo Cancer designation for aggressive cancers following C19 vaccines.
The final point to made is that both men presented in these case studies had evidence of active CoVax Disease in sites other than their aortas and hearts that would likely have progressed over time had they survived.