Report 45: Failure of Serialization By Pfizer Flouted Established Pharma Industry Rules


Introduction:
There are strict protocols in place regarding the storage and distribution of all pharmaceuticals to ensure safety throughout the delivery process. Particularly for mRNA Covid vaccines that were distributed by billions worldwide, those protocols should have been carefully practiced. Dr. Chris Flowers has reported that not only were standard protocols waived in confidential contracts between Pfizer and a variety of countries, but also the sensitive nature of the mRNA encased in lipid nanoparticles requires a variety of technical factors that are extremely challenging to consistently follow.
Additionally, there are legal requirements in place to ensure tracking and quality assurance of every single dose of vaccine. Generally, each dose should receive its own unique serial number, in addition to being assigned to a specific batch and a specific lot of vaccines. In the case of the Pfizer mRNA vaccines, five doses were batched in each vial, leaving the onsite staff to dilute and measure out each dose.
Please read the following detailed report by Dr. Flowers for DailyClout for a comprehensive explanation of serialization.
Failure of serialization by Pfizer flouted established pharmacuetical and Good Distribution Practice rules
Managing the quality of medical products as they are stored and distributed brings challenges with different storage requirements and expiry dates. As consumers, we cannot tell by sight or smell whether a drug has degraded during transport or been contaminated. Formalized Good Distribution Practices (GDP) are critical to the pharma industry, being essential to ensuring that when medicines are ready to be administered, patients can be confident they are effective, unadulterated and safe to use.
Pfizer actively disregarded both legislation and guidance required by various countries for distribution of the COVID vaccine, insisting on exclusion clauses in the contracts. Why did it do this and where is the quality control for a far-reaching intervention like a vaccine for the world population?
What legislation is there regarding good manufacturing and distribution practice of pharmaceuticals?
The Drug Quality and Security Act (DQSA) was enacted by Congress on November 27, 2013 [FDA, 2015. Drug Supply Chain Security Act. [Online] Available at: https://www.fda.gov/media/93779/download [Accessed 17 September 2022]]. This required interoperable, electronic tracing of products at the package level to identify and trace certain prescription drugs as they are distributed in the United States. Since November 2017, all pharmaceutical products were required to be serialized and compliant with the FDA’s guidance. ‘Track and Trace’ in the pharmaceuticals industry is now seen as a global mandate. Compliance deadlines have been put into place [Movilitas Engineering Group, n.d. DSCSA Compliance Deadlines and How to Prepare for Full Traceability. [Online] Available at: https://www.movilitas.com/insights/dscsa-compliance-deadlines-and-how-to-prepare-for-full-traceability/ [Accessed 17 September 2022]].
What is serialization and why is it important?
Serialization means that the manufacturer must apply a 2D barcode to every unit of finished product produced and then upload this manufacturing information to a central database. As the product moves through the distribution to the end user, the barcode is scanned and can be checked for authenticity. In a process with multiple steps (units), each with a barcode, quality control can easily be maintained.
What are the benefits of serialization?
Multiple benefits arise by following this process:
- Traceability – for each step of the manufacture
- An effective way to ensure brand authenticity and reduce batch recalls
- To assist with more efficient drug distribution
- Full compliance with government traceability regulations
- Potentially an end to counterfeit medicine
What exactly is supposed to be traced?
The FDA requires both a lot number and a batch number. Their definitions of these terms are as follows –
Batch means a specific quantity of a drug or other material that is intended to have uniform character and quality, within specified limits, and is produced according to a single manufacturing order during the same cycle of manufacture.
Lot number, control number, or batch number means any distinctive combination of letters, numbers, or symbols, or any combination of them, from which the complete history of the manufacture, processing, packing, holding, and distribution of a batch or lot of drug product or other material can be determined [FDA, 2022. CFR – Code of Federal Regulations Title 21. [Online]
Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=210.3
[Accessed 17 September 2022]].
What is Good Distribution Practice?
The U.S. Pharmacopeia (USP) is the source of many of the best practice guidelines (GxP) for distribution of products as regulated by the FDA. Similar documentation is provided by the United Kingdom’s Medicines and Healthcare Products Regulatory Authority (MHRA) and the European Medicines Agency (EMA) [MHRA (Medicines and Healthcare products Regulatory Agency) 2017, 2017. Rules and Guidance for Pharmaceutical Manufacturers and Distributors (The Orange Guide) eBook. [Online] Available at: https://www.pharmpress.com/product/9780857112910/orangeguide. [Accessed 17 September 2022]].
- Good Distribution Practice (GDP) is one of the four pillars of essential good practices required to ensure medicinal products are produced to the approved license, to remain safe, effective and of the requisite quality
- The other three pillars are Good Manufacturing Practice (GMP), Good Clinical Practice (GCP) and Good Laboratory Practice (GLP)
- Together, they make up the Pharmaceutical Quality Management System (QMS)
- GDP has become a critical element in the quality of medicinal products produced as supply chains have globalized and biologics, living things that are sensitive to environmental changes, have grown with the advent of monoclonal antibodies and gene therapies
- Regulatory authorities (FDA/EMA/MHRA) are required to inspect for compliance with GDP across all companies registered as a component of the clinical and/or commercial supply chain
There are many elements that make up Good Distribution Practice, and here are just a few of them –
- The Pharmaceutical Quality System
- Premises and Equipment
- Documentation
- Operations
- Complaints, Returns, Suspected Falsified Medicinal Products and Medicinal Product Recalls
- Self-Inspections
- Transportation
How does this relate to the COVID vaccine manufacture?
Most vaccines in the United States are provided as single-dose vials or pre-filled syringes, but the mRNA/lipid nanoparticle platform developed for this vaccine was packaged into multiple-dose vials for shipping around the world [Joshua Eaton, N. N., 2021. The U.S. is discarding millions of Covid vaccines. One cause: Multi-dose vials. [Online] Available at: https://www.nbcnews.com/news/us-news/u-s-discarding-millions-covid-vaccines-one-cause-multi-dose-n1279901. [Accessed 17 September 2022]]. This applies to both Pfizer and Moderna mRNA vaccine products.
Manufacture of the COVID vaccine is complex, a trade secret and has many components (i.e., inputs), which would have lot and batch numbers for each part. Quality control is a major issue given that mRNA is very unstable, as reported by the European Medicines Agency and published in the British Medical Journal (BMJ) [Serena Tinari, B., 2021. The EMA Covid-19 data leak, and what it tells us about mRNA instability. [Online] Available at: https://www.bmj.com/content/372/bmj.n627. [Accessed 17 September 2022]], and the LNP platform is tricky to get consistently right, both for the size of the particles and the distribution of mRNA within them [Christo T. Tzachev, H. L. S., 2012. Lipid Nanoparticles at the Current Stage and Prospects – A Review Article. [Online] Available at: https://www.globalresearchonline.net/journalcontents/v18-1/15.pdf. [Accessed 17 September 2022]].
Furthermore, there are technical issues with the mRNA/LNP platform which require ultra-low-temperature freezers to maintain the integrity of these lipid particles, as they are subject to oxidative degradation where the lipids form into clumps. Indeed, there are many issues with the LNP storage and transport, as they can be easily destroyed by vigorous shaking, including agitation from road transport.
At the start of COVID vaccine production, the where and how of mRNA manufacturing was a source of controversy, and documentation of the early days is not readily available. At a certain date, Pfizer started a group of factories within the US making the different stages of the vaccine: Chesterfield, Missouri, where the antigen DNA was manufactured; then Andover, Massachusetts, where mRNA was made; followed by Portage, Michigan, where the LNPs are combined with the mRNA, which takes around four days [Elizabeth Weise, K. W., 2021. A COVID-19 vaccine life cycle: from DNA to doses. [Online] Available at: https://eu.usatoday.com/in-depth/news/health/2021/02/07/how-covid-vaccine-made-step-step-journey-pfizer-dose/4371693001/. [Accessed 17 September 2022]]. After that, they were combined into the LNPs and packaged into five-dose vials. This is the ‘finalized’ product leaving the manufacturer, which the rules require to be serialized with a barcode.
Serialization requires barcoding for every finalized dose of medicine, and each individual dose should have been given a lot and batch number. But, this could not possibly happen with either the Pfizer or Moderna vaccines, because they left the manufacturer frozen and in vials containing five or six doses, rather than single doses. Furthermore, each separate dose of the vaccines was not done by the manufacturer. Rather, it was finalized on-site by diluting the five-dose vial with saline and drawing each dose up into individual syringes for injection.
Questions have been raised regarding the monitoring of quality control of COVID vaccine manufacture, which is not just a Pfizer issue, as other manufacturers had bad batches that had to be withdrawn due to contamination. Here are two examples –
- 60 million doses of Johnson and Johnson vaccine made at its Baltimore plant had to be withdrawn [Burroughs, D., 2021. FDA Finds 60 Million COVID Vaccine Doses Were Potentially Contaminated: Report. [Online] Available at: https://www.westernjournal.com/fda-finds-60-million-covid-vaccine-doses-potentially-contaminated-report/. [Accessed 17 September 2022]].
- Another example was in Japan, where a batch of Moderna mRNA vaccine had to be recalled due to apparent contamination [Guenot, M., 2021. Japan investigating whether 3 deaths are linked to a Moderna vaccine batch that officials fear was contaminated. [Online] Available at: https://www.businessinsider.com/three-dead-recalled-contaminated-batch-investigation-japan-moderna-2021-9?op=1&r=US&IR=T. [Accessed 17 September 2022]].
How did we learn that there was a contractual issue with serialization and Pfizer?
This revelation happened due to the leaking of an unredacted contract between Pfizer and the European Union (EU). [https://maloneinstitute.org/pfizer-biontech] Originally reported by Reuters and multiple news media in April 2021, Pfizer had 73 formalized deals with countries around the world for its COVID-19 vaccine at that time. But, of those, only five had been published by governments, and they included ‘significant redactions.’ Apart from charging different prices in different countries, they also included the phrase, ‘the Participating Member State acknowledges that the Vaccine shall not be serialized.’ The contract between Pfizer and the European Union was termed an Advance Purchase Agreement [Pfizer, E. C. a., 2021. Contract Between the European Commission And Pfizer (Manufacturing And Supply Agreement). [Online] Available at: https://archive.org/details/contract_03. [Accessed 17 September 2022]].
The contract between the European Commission and Pfizer was leaked in March 2021 to a Belgian association, Notre Bon Droit.

Title page of Contract Between the European Commission and Pfizer

Contract Between the European Commission and Pfizer – pp. 48 and 49
This piqued the interest of members of the European Parliament, who noted unusual legal requests by Pfizer in the contract, and they made formal requests for information [(ID), G. R., 2021. Parliamentary question – E-002296/2021. [Online] Available at: https://www.europarl.europa.eu/doceo/document/E-9-2021-002296_EN.html. [Accessed 17 September 2022]]. The narrative shared by news media focused on different pricing between jurisdictions and lack of accountability and limitations of liability. The unusual legal requests about formal serialization were overlooked.
How do the GDP (distribution) rules intersect with the absence of serialization of the vaccine?
All components of the COVID vaccine should have been given both lot and batch numbers during manufacture, and, once the mRNA was incorporated into the lipid nanoparticles and placed into vials, they should have been assigned a serialized barcode, according to standard practice.
When it comes to distribution, barcodes are required to manage the safe flow of the product to its destination. Licensed wholesale distributors must comply with GDP, but, uniquely for this type of vaccine, they had to be stored and shipped at ultra-low temperatures (deep frozen down to minus 112 degrees Fahrenheit) and protected from vibration. Due to the uniqueness of this platform, the distribution networks were inadequate, and an alternative was used, bypassing the normal, regulated networks of distribution. As a result, by using a novel distribution method rather than the normal wholesale distribution network, the vaccine escaped the safety mechanisms that other pharmaceuticals are mandated to follow.
Are there other potential issues that arise with a multi-dose, LNP vaccine when it is time to be administered?
First described in the clinical trials protocol, and later in the instructions for use in the commercial product, there were strict instructions for use, with which most medical staff would be unfamiliar, compared with a regular injection.
The vials had to be stored locally in a freezer, and then the vials had to be thawed within a strict usage window of two weeks. Before use, the vials had to be thawed, mixed with saline, and inverted gently 10 times before use – not shaken – and then discarded after six hours [DailyMed, 2022. LABEL: COMIRNATY- covid-19 vaccine, mrna injection, suspension. [Online] Available at: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=48c86164-de07-4041-b9dc-f2b5744714e5#section-2.1. [Accessed 17 September 2022]].
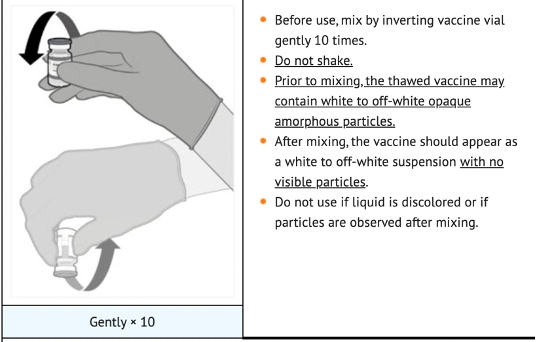
The gentle inversion allowed mixing of the saline and LNPs to make a smooth, white suspension. If the right amount of inversion had not been performed, then each dose could have a different concentration of mRNA. If the vials were shaken, there was a chance that the LNPs would have been disrupted, and some LNPs may not contain mRNA and others may contain a higher dose.
What does all this mean for us?
Unlike normal regulated pharmaceutical products, the multi-dose vaccine does not have the basic manufacturing information and required codes needed to provide the expected quality control of a pharma product, including consistency in dosing, due to requirements of not having a finalized product.
If we are to trust vaccine manufacturers in the future, good quality control needs to be established, as exist with other medical products, with full transparency of the ingredients and potential adverse effects, including severe ones, which will allow recipients to give informed consent.
The use of multi-dose vaccine vials which need reconstituting with saline should cease; and barcoded, single-use, pre-filled syringes should be standard practice.





This article was written following the information published in ‘InsidePharma’ Substack, by Hedley Rees. (https://hedleyrees.substack.com/) He also educated me and provided me with some material about Good Distribution Practice, which was not referenced in the study